Key Points
-
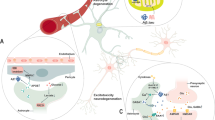
Combined molecular and functional analyses have revealed that astrocytes are direct, active communication partners of neurons. They comprise a heterogeneous group of cells that are distinguished by their morphology, molecular assembly, functional characteristics and regional distribution within the CNS. Defective astrocytes are involved in various neurological disorders. Dysfunction of glial glutamate transporters seems to represent a spanning phenomenon common to many pathological conditions.
-
The hippocampus of patients with temporal lobe epilepsy shows severe histopathological abnormalities. Recent studies showed that in the sclerotic hippocampus dislocation of water channels (aquaporin 4; AQP4) and reduced expression of inwardly rectifying K+ (Kir) channels in astrocytes could contribute to impaired K+ buffering and increased seizure propensity. Moreover, dysregulation of glial glutamate uptake, slowed glutamate–glutamine cycling and subsequent extracellular transmitter accumulation seem to contribute to seizure generation in hippocampal sclerosis.
-
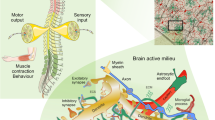
Astrocytes contribute to the pathology of sporadic amyotrophic lateral sclerosis (ALS). Dysfunction of glial glutamate transporters causes increases in extracellular glutamate concentrations and excitotoxic neuronal damage. In hereditary forms of ALS, mutations of Cu/Zn superoxide dismutase (SOD1) lead to oxidative stress and aberrant biochemistry in motor neurons and astrocytes. Apparently, fatal dysregulation of neuron–glia interactions leads to neurotoxicity through the release of reactive oxygen species, prostaglandins, mutant SOD1 and neurotransmitters.
-
The realization that several membrane proteins are highly segregated in astrocytes, with AQP4 and Kir channels being prominent examples, provides a molecular basis to view astrocytes as fundamental regulators of neurovascular units. Redistribution and functional impairment of these molecules, which is seen following ischaemia and stroke, suggests that they represent potential targets for therapeutic interventions. Moreover, astrocytes are becoming increasingly recognized as the source of a plethora of regulatory molecules that influence neurogenesis, neuritogenesis and vasculogenesis, and, as such, could function as orchestrators of these processes in development and regeneration.
-
Hepatic insufficiency leads to hyperammonaemia and elevated concentrations of ammonia in cerebrospinal fluid. Enhanced uptake of ammonia/ammonium ions by astrocytes affects the glutamate–glutamine cycle, resulting in swelling, perturbed K+ homeostasis and reduced glutamate uptake. Although the role of astrocytes in hepatic encephalopathy has been well studied, a useful molecular target to prevent their dysfunction has not yet been identified.
-
Although a vast body of evidence documents astroglial dysfunction and dysregulation of astroglia-specific functions in various diseases, it seems premature to try to construe a unifying picture from these data. In particular, it is still often unclear whether glial changes are causative of a given disease or represent an accompanying phenomenon. A better definition of astroglial subtypes should promote our understanding of their specific roles in pathophysiology and the development of cell-centred therapeutic approaches.
Abstract
Recent work on glial cell physiology has revealed that glial cells, and astrocytes in particular, are much more actively involved in brain information processing than previously thought. This finding has stimulated the view that the active brain should no longer be regarded solely as a network of neuronal contacts, but instead as a circuit of integrated, interactive neurons and glial cells. Consequently, glial cells could also have as yet unexpected roles in the diseased brain. An improved understanding of astrocyte biology and heterogeneity and the involvement of these cells in pathogenesis offers the potential for developing novel strategies to treat neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Parpura, V. et al. Glutamate-mediated astrocyte–neuron signalling. Nature 369, 744–747 (1994).
Steinhäuser, C., Jabs, R. & Kettenmann, H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus 4, 19–35 (1994).
Jabs, R. et al. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J. Cell Sci. 118, 3791–3803 (2005).
Matthias, K. et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J. Neurosci. 23, 1750–1758 (2003).
Wallraff, A., Odermatt, B., Willecke, K. & Steinhäuser, C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia 48, 36–43 (2004).
Verkhratsky, A. & Steinhäuser, C. Ion channels in glial cells. Brain Res. Rev. 32, 380–412 (2000).
Kettenmann, H. & Steinhäuser, C. In Neuroglia 2nd edn (eds Kettenmann, H. & Ransom, B. R.) 131–145 (Oxford Univ. Press, 2005).
Higashi, K. et al. An inwardly rectifying K+ channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am. J. Physiol. Cell Physiol. 281, C922–C931 (2001).
Verkman, A. S. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. 118, 3225–3232 (2005).
Amiry-Moghaddam, M., Frydenlund, D. S. & Ottersen, O. P. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 129, 999–1010 (2004).
Theis, M., Sohl, G., Eiberger, J. & Willecke, K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 28, 188–195 (2005).
Nedergaard, M., Ransom, B. & Goldman, S. A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26, 523–530 (2003).
Danbolt, N. C. Glutamate uptake. Prog. Neurobiol. 65, 1–105 (2001).
Bergles, D. E., Diamond, J. S. & Jahr, C. E. Clearance of glutamate inside the synapse and beyond. Curr. Opin. Neurobiol. 9, 293–298 (1999).
Volterra, A. & Meldolesi, J. Astrocytes, from brain glue to communication elements: the revolution continues. Nature Rev. Neurosci. 6, 626–640 (2005).
Zonta, M. et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neurosci. 6, 43–50 (2003).
Mulligan, S. J. & MacVicar, B. A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004).
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nature Neurosci. 9, 260–267 (2006).
Kim, J. H. Pathology of epilepsy. Exp. Mol. Pathol. 70, 345–367 (2001).
Blümcke, I., Thom, M. & Wiestler, O. D. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 12, 199–211 (2002).
De Lanerolle, N. C. & Lee, T. S. New facets of the neuropathology and molecular profile of human temporal lobe epilepsy. Epilepsy Behav. 7, 190–203 (2005).
Kivi, A. et al. Effects of barium on stimulus-induced rises of [K+]o in human epileptic non-sclerotic and sclerotic hippocampal area CA1. Eur. J. Neurosci. 12, 2039–2048 (2000).
Hinterkeuser, S. et al. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur. J. Neurosci. 12, 2087–2096 (2000). References 22 and 23 show the downregulation of inwardly rectifying K+ currents and the impairment of Ba2+-sensitive K+-buffering in the sclerotic hippocampus from epilepsy patients.
Bordey, A. & Sontheimer, H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 32, 286–303 (1998).
Schröder, W. et al. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia 41, S181–S184 (2000).
Binder, D. K. et al. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia (in the press).
Eid, T. et al. Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc. Natl Acad. Sci. USA 102, 1193–1198 (2005). Reports that in patients with AHS the density of AQP4 water channels at perivascular end-feet is reduced. The perturbed water flux through astrocytes entails impaired buffering of extracellular potassium.
Amiry-Moghaddam, M. et al. An α-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl Acad. Sci. USA 100, 2106–2111 (2003). Using a mouse model with defective anchoring of AQP4 to the cytoskeleton, the authors document the importance of proper subcellular segregation for aquaporin function in astrocytes under ischaemic stress in vivo.
Glass, M. & Dragunow, M. Neurochemical and morphological changes associated with human epilepsy. Brain Res. Rev. 21, 29–41 (1995).
Tanaka, K. et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 (1997).
Rothstein, J. D. et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 (1996).
Campbell, S. L. & Hablitz, J. J. Glutamate transporters regulate excitability in local networks in rat neocortex. Neuroscience 127, 625–635 (2004).
Demarque, M. et al. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J. Neurosci. 24, 3289–3294 (2004).
Wong, M. et al. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann. Neurol. 54, 251–256 (2003).
Tessler, S., Danbolt, N. C., Faull, R. L. M., Storm-Mathisen, J. & Emson, P. C. Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience 88, 1083–1091 (1999).
Mathern, G. W. et al. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology 52, 453–472 (1999).
Proper, E. A. et al. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 125, 32–43 (2002).
During, M. J. & Spencer, D. D. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341, 1607–1610 (1993).
Eid, T. et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 363, 28–37 (2004).
Petroff, O. A., Errante, L. D., Rothman, D. L., Kim, J. H. & Spencer, D. D. Glutamate–glutamine cycling in the epileptic human hippocampus. Epilepsia 43, 703–710 (2002). References 39 and 40 indicate that in epilepsy in humans downregulation of astroglial glutamine synthetase disturbs the conversion of glutamate and its accumulation in the extracellular space.
Rothstein, J. D. et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433, 73–77 (2005). Identifies β-lactam antibiotics as potential therapeutic agents for the treatment of diseases associated with excitotoxic degeneration through upregulation of the astroglial glutamate transporter EAAT2.
Brusa, R. et al. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science 270, 1677–1680 (1995).
Seifert, G. et al. Changes in flip/flop splicing of astroglial AMPA receptors in human temporal lobe epilepsy. Epilepsia 43 (Suppl. 5), 162–167 (2002).
Seifert, G., Hüttmann, K., Schramm, J. & Steinhäuser, C. Enhanced relative expression of glutamate receptor 1 flip AMPA receptor subunits in hippocampal astrocytes of epilepsy patients with Ammon's horn sclerosis. J. Neurosci. 24, 1996–2003 (2004). The authors show that aberrant AMPA receptor splicing in astrocytes of the sclerotic human hippocampus affects the kinetics of AMPA receptor desensitization, which might contribute to seizure generation.
Schröder, W., Seifert, G., Hüttmann, K., Hinterkeuser, S. & Steinhäuser, C. AMPA receptor-mediated modulation of inward rectifier K+ channels in astrocytes of mouse hippocampus. Mol. Cell Neurosci. 19, 447–458 (2002).
Whitney, K. D. & McNamara, J. O. GluR3 autoantibodies destroy neural cells in a complement-dependent manner modulated by complement regulatory proteins. J. Neurosci. 20, 7307–7316 (2000).
Manning, T. J. Jr & Sontheimer, H. Spontaneous intracellular calcium oscillations in cortical astrocytes from a patient with intractable childhood epilepsy (Rasmussen's encephalitis). Glia 21, 332–337 (1997).
Steinhäuser, C. & Seifert, G. Glial membrane channels and receptors in epilepsy: impact for generation and spread of seizure activity. Eur. J. Pharmacol. 447, 227–237 (2002).
Tang, F. R. & Lee, W. L. Expression of the group II and III metabotropic glutamate receptors in the hippocampus of patients with mesial temporal lobe epilepsy. J. Neurocytol. 30, 137–143 (2001).
Aronica, E. et al. Expression and cell distribution of group I and group II metabotropic glutamate receptor subtypes in taylor-type focal cortical dysplasia. Epilepsia 44, 785–795 (2003).
Aronica, E. et al. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur. J. Neurosci. 17, 2106–2118 (2003).
Kang, N., Xu, J., Xu, Q., Nedergaard, M. & Kang, J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 94, 4121–4130 (2005).
Tian, G. F. et al. An astrocytic basis of epilepsy. Nature Med. 11, 973–981 (2005). Reports that, in acute epilepsy models, glutamate released from astrocytes causes paroxysmal depolarization shifts in neurons. Several antiepileptic drugs were shown to reduce astrocytic [Ca2+]i elevations and therefore astrocytes might be considered as new targets for antiepileptic treatments.
Rothstein, J. D., Martin, L. J. & Kuncl, R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 326, 1464–1468 (1992).
Rothstein, J. D., Van Kammen, M., Levey, A. I., Martin, L. J. & Kuncl, R. W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 38, 73–84 (1995). References 54 and 55 describe the loss of glial glutamate transporters in ALS and identify astrocytes as crucial mediators of excitotoxicity and motor neuron loss.
Sasaki, S., Komori, T. & Iwata, M. Excitatory amino acid transporter 1 and 2 immunoreactivity in the spinal cord in amyotrophic lateral sclerosis. Acta Neuropathol. (Berl.) 100, 138–144 (2000).
Yamada, K. et al. Glutamate transporter GLT-1 is transiently localized on growing axons of the mouse spinal cord before establishing astrocytic expression. J. Neurosci. 18, 5706–5713 (1998).
Schmitt, A., Asan, E., Puschel, B., Jons, T. & Kugler, P. Expression of the glutamate transporter GLT1 in neural cells of the rat central nervous system: non-radioactive in situ hybridization and comparative immunocytochemistry. Neuroscience 71, 989–1004 (1996).
Lin, C. L. et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20, 589–602 (1998). Shows that in patients with ALS, loss of astroglial EAAT2 is due to aberrant RNA splicing.
Meyer, T. et al. The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. J. Neurol. Sci. 170, 45–50 (1999).
Honig, L. S., Chambliss, D. D., Bigio, E. H., Carroll, S. L. & Elliott, J. L. Glutamate transporter EAAT2 splice variants occur not only in ALS, but also in AD and controls. Neurology 55, 1082–1088 (2000).
Volterra, A., Trotti, D., Tromba, C., Floridi, S. & Racagni, G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J. Neurosci. 14, 2924–2932 (1994).
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993). First report that mutation of the Cu/Zn superoxide dismutase ( SOD1 ) gene mediates neurodegeneration in hereditary ALS.
Trotti, D., Rolfs, A., Danbolt, N. C., Brown, R. H. Jr & Hediger, M. A. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nature Neurosci. 2, 427–433 (1999). Shows that mutant SOD1 leads to neurotoxicity through inactivation of the astroglial glutamate transporter EAAT2.
Howland, D. S. et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc. Natl Acad. Sci. USA 99, 1604–1609 (2002).
Bruijn, L. I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Pedersen, W. A. et al. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann. Neurol. 44, 819–824 (1998).
Bruijn, L. I., Miller, T. M. & Cleveland, D. W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 (2004).
Pramatarova, A., Laganiere, J., Roussel, J., Brisebois, K. & Rouleau, G. A. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 21, 3369–3374 (2001).
Lino, M. M., Schneider, C. & Caroni, P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J. Neurosci. 22, 4825–4832 (2002).
Gong, Y. H., Parsadanian, A. S., Andreeva, A., Snider, W. D. & Elliott, J. L. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. 20, 660–665 (2000).
Clement, A. M. et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 (2003).
Urushitani, M. et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nature Neurosci. 9, 108–118 (2006). Suggests that, in ALS, astrocytes contribute to the secretion of mutant SOD1, which, in turn, produces microgliosis and motor neuron death.
Rao, S. D., Yin, H. Z. & Weiss, J. H. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J. Neurosci. 23, 2627–2633 (2003).
Rao, S. D. & Weiss, J. H. Excitotoxic and oxidative cross-talk between motor neurons and glia in ALS pathogenesis. Trends Neurosci. 27, 17–23 (2004).
Levine, J. B., Kong, J. M., Nadler, M. & Xu, Z. S. Astrocytes interact intimately with degenerating motor neurons in mouse amyotrophic lateral sclerosis (ALS). Glia 28, 215–224 (1999).
Almer, G. et al. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann. Neurol. 49, 176–185 (2001).
Yasojima, K., Tourtellotte, W. W., McGeer, E. G. & McGeer, P. L. Marked increase in cyclooxygenase-2 in ALS spinal cord: implications for therapy. Neurology 57, 952–956 (2001).
Yamagata, K., Andreasson, K. I., Kaufmann, W. E., Barnes, C. A. & Worley, P. F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11, 371–386 (1993).
Pepicelli, O. et al. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E2 extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J. Neurochem. 81, 1028–1034 (2002).
Bezzi, P. et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391, 281–285 (1998).
Drachman, D. B. et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann. Neurol. 52, 771–778 (2002).
Aronica, E., Catania, M. V., Geurts, J., Yankaya, B. & Troost, D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neuroscience 105, 509–520 (2001).
Catania, M. V., Aronica, E., Yankaya, B. & Troost, D. Increased expression of neuronal nitric oxide synthase spliced variants in reactive astrocytes of amyotrophic lateral sclerosis human spinal cord. J. Neurosci. 21, RC148 (2001). Suggests that in human ALS aberrant expression of neuronal NOS in reactive astrocytes is involved in pathogenesis.
Trotti, D. et al. Peroxynitrite inhibits glutamate transporter subtypes. J. Biol. Chem. 271, 5976–5979 (1996).
Beal, M. F. et al. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 42, 644–654 (1997).
Bruno, V. et al. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J. Cereb. Blood Flow Metab. 21, 1013–1033 (2001).
Amiry-Moghaddam, M. et al. α-Syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood–brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 18, 542–544 (2004).
Manley, G. T. et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nature Med. 6, 159–163 (2000).
Lo, A. C. et al. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J. Cereb. Blood Flow Metab. 25, 998–1011 (2005).
Da, T. & Verkman, A. S. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Invest. Ophthalmol. Vis. Sci. 45, 4477–4483 (2004).
Dalloz, C. et al. Targeted inactivation of dystrophin gene product Dp71: phenotypic impact in mouse retina. Hum. Mol. Genet. 12, 1543–1554 (2003).
Hibino, H., Fujita, A., Iwai, K., Yamada, M. & Kurachi, Y. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J. Biol. Chem. 279, 44065–44073 (2004).
Thomzig, A. et al. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K–ATP channels. Mol. Cell. Neurosci. 18, 671–690 (2001).
Stonehouse, A. H. et al. Characterisation of Kir2.0 proteins in the rat cerebellum and hippocampus by polyclonal antibodies. Histochem. Cell Biol. 112, 457–465 (1999).
Farahani, R. et al. Alterations in metabolism and gap junction expression may determine the role of astrocytes as 'good samaritans' or executioners. Glia 50, 351–361 (2005).
Nakase, T., Söhl, G., Theis, M., Willecke, K. & Naus, C. C. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am. J. Pathol. 164, 2067–2075 (2004).
Perez Velazquez, J. L., Kokarovtseva, L., Sarbaziha, R., Jeyapalan, Z. & Leshchenko, Y. Role of gap junctional coupling in astrocytic networks in the determination of global ischaemia-induced oxidative stress and hippocampal damage. Eur. J. Neurosci. 23, 1–10 (2006).
Nicchia, G. P. et al. New possible roles for aquaporin-4 in astrocytes: cell cytoskeleton and functional relationship with connexin43. FASEB J. 19, 1674–1676 (2005).
Rozyczka, J., Figiel, M. & Engele, J. Chronic endothelin exposure inhibits connexin43 expression in cultured cortical astroglia. J. Neurosci. Res. 79, 303–309 (2005).
Lin, J. H. et al. Connexin mediates gap junction-independent resistance to cellular injury. J. Neurosci. 23, 430–441 (2003).
Takano, T. et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc. Natl Acad. Sci. USA 102, 16466–16471 (2005).
Ye, Z. C., Wyeth, M. S., Baltan-Tekkok, S. & Ransom, B. R. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 23, 3588–3596 (2003).
Fujita, H., Sato, K., Wen, T. C., Peng, Y. & Sakanaka, M. Differential expressions of glycine transporter 1 and three glutamate transporter mRNA in the hippocampus of gerbils with transient forebrain ischemia. J. Cereb. Blood Flow Metab. 19, 604–615 (1999).
Maragakis, N. J. & Rothstein, J. D. Glutamate transporters in neurologic disease. Arch. Neurol. 58, 365–370 (2001).
Hamann, M., Rossi, D. J., Marie, H. & Attwell, D. Knocking out the glial glutamate transporter GLT-1 reduces glutamate uptake but does not affect hippocampal glutamate dynamics in early simulated ischaemia. Eur. J. Neurosci. 15, 308–314 (2002).
Mitani, A. & Tanaka, K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J. Neurosci. 23, 7176–7182 (2003).
Rao, V. L. et al. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J. Neurosci. 21, 1876–1883 (2001).
Selkirk, J. V. et al. Role of the GLT-1 subtype of glutamate transporter in glutamate homeostasis: the GLT-1-preferring inhibitor WAY-855 produces marginal neurotoxicity in the rat hippocampus. Eur. J. Neurosci. 21, 3217–3228 (2005).
Swanson, R. A. et al. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci. 17, 932–940 (1997).
Schluter, K., Figiel, M., Rozyczka, J. & Engele, J. CNS region-specific regulation of glial glutamate transporter expression. Eur. J. Neurosci. 16, 836–842 (2002).
Reagan, L. P. et al. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc. Natl Acad. Sci. USA 101, 2179–2184 (2004).
Zschocke, J. et al. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J. Biol. Chem. 280, 34924–34932 (2005).
Feustel, P. J., Jin, Y. & Kimelberg, H. K. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke 35, 1164–1168 (2004).
Yamamoto-Mizuma, S. et al. Altered properties of volume-sensitive osmolyte and anion channels (VSOACs) and membrane protein expression in cardiac and smooth muscle myocytes from Clcn3−/− mice. J. Physiol. 557, 439–456 (2004).
Jentsch, T. J., Stein, V., Weinreich, F. & Zdebik, A. A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 82, 503–568 (2002).
Kimelberg, H. K. et al. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport 11, 2675–2679 (2000).
Zhang, Y. et al. Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp. Neurol. 196, 41–46 (2005).
Osuka, K., Feustel, P. J., Mongin, A. A., Tranmer, B. I. & Kimelberg, H. K. Tamoxifen inhibits nitrotyrosine formation after reversible middle cerebral artery occlusion in the rat. J. Neurochem. 76, 1842–1850 (2001). Suggests that tamoxifen is an inhibitor of Ca2+/calmodulin-dependent NOS and the subsequent peroxynitrite production in transient focal cerebral ischaemia, which might represent one mechanism for the neuroprotective effect of tamoxifen.
Kimelberg, H. K. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 50, 389–397 (2005).
Williams, S. M. et al. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia 49, 520–541 (2005).
Serini, G. & Bussolino, F. Common cues in vascular and axon guidance. Physiology (Bethesda) 19, 348–354 (2004).
Carmeliet, P. & Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005).
Tsang, M. C., Lo, A. C., Cheung, P. T., Chung, S. S. & Chung, S. K. Perinatal hypoxia-/ischemia-induced endothelin-1 mRNA in astrocyte-like and endothelial cells. Neuroreport 12, 2265–2270 (2001).
Ehrenreich, H. The astrocytic endothelin system: toward solving a mystery focus on 'distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca2+ signaling'. Am. J. Physiol. 277, C614–C615 (1999).
Acker, T., Beck, H. & Plate, K. H. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech. Dev. 108, 45–57 (2001).
West, H., Richardson, W. D. & Fruttiger, M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development 132, 1855–1862 (2005).
Sun, Y. et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 111, 1843–1851 (2003).
Zhang, H., Vutskits, L., Pepper, M. S. & Kiss, J. Z. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J. Cell Biol. 163, 1375–1384 (2003).
Kosacka, J. et al. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 320, 11–19 (2005).
Ward, N. L., Putoczki, T., Mearow, K., Ivanco, T. L. & Dumont, D. J. Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J. Comp. Neurol. 482, 244–256 (2005).
Danielyan, L. et al. The blockade of endothelin A receptor protects astrocytes against hypoxic injury: common effects of BQ-123 and erythropoietin on the rejuvenation of the astrocyte population. Eur. J. Cell Biol. 84, 567–579 (2005).
Fagan, S. C., Hess, D. C., Hohnadel, E. J., Pollock, D. M. & Ergul, A. Targets for vascular protection after acute ischemic stroke. Stroke 35, 2220–2225 (2004).
Ott, P. & Larsen, F. S. Blood–brain barrier permeability to ammonia in liver failure: a critical reappraisal. Neurochem. Int. 44, 185–198 (2004).
Takahashi, H., Koehler, R. C., Brusilow, S. W. & Traystman, R. J. Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am. J. Physiol. 261, H825–H829 (1991).
Willard-Mack, C. L. et al. Inhibition of glutamine synthetase reduces ammonia-induced astrocyte swelling in rat. Neuroscience 71, 589–599 (1996).
Suarez, I., Bodega, G. & Fernandez, B. Glutamine synthetase in brain: effect of ammonia. Neurochem. Int. 41, 123–142 (2002).
Butterworth, R. F. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab. Brain Dis. 17, 221–227 (2002).
Sugimoto, H., Koehler, R. C., Wilson, D. A., Brusilow, S. W. & Traystman, R. J. Methionine sulfoximine, a glutamine synthetase inhibitor, attenuates increased extracellular potassium activity during acute hyperammonemia. J. Cereb. Blood Flow Metab. 17, 44–49 (1997).
Rama Rao, K. V., Chen, M., Simard, J. M. & Norenberg, M. D. Increased aquaporin-4 expression in ammonia-treated cultured astrocytes. Neuroreport 14, 2379–2382 (2003).
Bender, A. S. & Norenberg, M. D. Effects of ammonia on L-glutamate uptake in cultured astrocytes. Neurochem. Res. 21, 567–573 (1996).
Norenberg, M. D., Huo, Z., Neary, J. T. & Roig-Cantesano, A. The glial glutamate transporter in hyperammonemia and hepatic encephalopathy: relation to energy metabolism and glutamatergic neurotransmission. Glia 21, 124–133 (1997).
Knecht, K., Michalak, A., Rose, C., Rothstein, J. D. & Butterworth, R. F. Decreased glutamate transporter (GLT-1) expression in frontal cortex of rats with acute liver failure. Neurosci. Lett. 229, 201–203 (1997).
Watanabe, A. et al. Glutamic acid and glutamine levels in serum and cerebrospinal fluid in hepatic encephalopathy. Biochem. Med. 32, 225–231 (1984).
Michalak, A., Rose, C., Butterworth, J. & Butterworth, R. F. Neuroactive amino acids and glutamate (NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology 24, 908–913 (1996).
Monfort, P., Munoz, M. D., ElAyadi, A., Kosenko, E. & Felipo, V. Effects of hyperammonemia and liver failure on glutamatergic neurotransmission. Metab. Brain Dis. 17, 237–250 (2002).
Rose, C., Kresse, W. & Kettenmann, H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J. Biol. Chem. 280, 20937–20944 (2005). Shows that hyperammonaemia produces an increase in [Ca2+]i and triggers glial glutamate release in cultured astrocytes. This mechanism might contribute to disorders associated with glutamate-dependent overexcitation.
Szerb, J. C. & Butterworth, R. F. Effect of ammonium ions on synaptic transmission in the mammalian central nervous system. Prog. Neurobiol. 39, 135–153 (1992).
Michalak, A. & Butterworth, R. F. Selective loss of binding sites for the glutamate receptor ligands [3H]kainate and (S)-[3H]5-fluorowillardiine in the brains of rats with acute liver failure. Hepatology 25, 631–635 (1997).
Götz, M. Glial cells generate neurons — master control within CNS regions: developmental perspectives on neural stem cells. Neuroscientist 9, 379–397 (2003).
Eichmann, A., Makinen, T. & Alitalo, K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 19, 1013–1021 (2005).
Binder, D. K., Papadopoulos, M. C., Haggie, P. M. & Verkman, A. S. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J. Neurosci. 24, 8049–8056 (2004).
Uckermann, O. et al. Glutamate-evoked alterations of glial and neuronal cell morphology in the guinea pig retina. J. Neurosci. 24, 10149–10158 (2004).
Izumi, Y. et al. Swelling of Müller cells induced by AP3 and glutamate transport substrates in rat retina. Glia 17, 285–293 (1996).
MacVicar, B. A., Feighan, D., Brown, A. & Ransom, B. Intrinsic optical signals in the rat optic nerve: role for K+ uptake via NKCC1 and swelling of astrocytes. Glia 37, 114–123 (2002).
Strange, K. Cellular volume homeostasis. Adv. Physiol. Educ. 28, 155–159 (2004).
Parkerson, K. A. & Sontheimer, H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia 46, 419–436 (2004).
Nielsen, S. et al. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 17, 171–180 (1997).
Nagelhus, E. A. et al. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 26, 47–54 (1999).
Niermann, H., Amiry-Moghaddam, M., Holthoff, K., Witte, O. W. & Ottersen, O. P. A novel role of vasopressin in the brain: modulation of activity-dependent water flux in the neocortex. J. Neurosci. 21, 3045–3051 (2001).
Amiry-Moghaddam, M. et al. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of α-syntrophin-null mice. Proc. Natl Acad. Sci. USA 100, 13615–13620 (2003).
Nagelhus, E. A., Mathiisen, T. M. & Ottersen, O. P. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 129, 905–913 (2004).
Papadopoulos, M. C. & Verkman, A. S. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 280, 13906–13912 (2005).
Bloch, O., Papadopoulos, M. C., Manley, G. T. & Verkman, A. S. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J. Neurochem. 95, 254–262 (2005).
Simard, M., Arcuino, G., Takano, T., Liu, Q. S. & Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 23, 9254–9262 (2003).
Acknowledgements
The authors wish to thank D. Binder for comments on the manuscript. Work of the authors is supported by Deutsche Forschungsgemeinschaft. We apologize to all those whose work could not be discussed due to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Complex glial cells
-
Cells in the postnatal brain that express various types of voltage- and time-dependent ion channels as well as transmitter receptors. They do not generate action potentials and have been shown to express S100β.
- GluR-type glial cells
-
Cells in the postnatal hippocampus that express voltage- and time-dependent ion channels and glutamate receptors of the AMPA type. These cells receive synaptic input from glutamatergic and GABA-containing neurons, and lack gap junctional coupling as well as functional glutamate transporters. They express glial fibrillary acidic protein transcripts and S100β protein and probably represent a subpopulation of the complex glial cells.
- Gap junctions
-
Gap junctions are channels composed of two juxtaposed hexameric hemichannels (connexons) forming intercellular pathways for the diffusion of ions and small molecules.
- Inwardly rectifying K+ channels
-
(Kir channels). These comprise a large family of transmembrane proteins that allow preferred passage of K+ at negative membrane potentials.
- Müller glia
-
A radial-shaped type of astroglial cell that spans the entire thickness of the retina and tightly envelops retinal neurons and neuronal processes. It constitutes the majority of retinal astroglia and is central to retinal development and function.
- Ammon's horn sclerosis
-
(AHS). A histopathological condition often associated with temporal lobe epilepsy, characterized by selective neuronal cell loss in the CA1 and CA4 regions of the hippocampus and reactive sclerosis.
- Stroke
-
Tissue damage in the CNS resulting from a lack of adequate blood supply. Stroke is a major cause of death and permanent disability.
- Neurovascular unit
-
Describes a dynamic ensemble comprising neurons, astroglial cells, vascular endothelia, and their associated extracellular matrices. The neurovascular unit is viewed as a modular unit integrating CNS function and perfusion (blood supply).
- Volume-sensitive osmolyte and anion channel
-
(VSOAC; also known as VRAC). A molecularly so far undefined channel that, on cell swelling, results in an outward rectifying, electrogenic flow of Cl- and mediates a passive efflux of osmolytes.
Rights and permissions
About this article
Cite this article
Seifert, G., Schilling, K. & Steinhäuser, C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7, 194–206 (2006). https://doi.org/10.1038/nrn1870
Issue Date:
DOI: https://doi.org/10.1038/nrn1870
This article is cited by
-
Regulatory T cells decrease C3-positive reactive astrocytes in Alzheimer-like pathology
Journal of Neuroinflammation (2023)
-
Extracellular vesicles released from macrophages modulates interleukin-1β in astrocytic and neuronal cells
Scientific Reports (2023)
-
Lipocalin-2: a therapeutic target to overcome neurodegenerative diseases by regulating reactive astrogliosis
Experimental & Molecular Medicine (2023)
-
Rapid degeneration of iPSC-derived motor neurons lacking Gdap1 engages a mitochondrial-sustained innate immune response
Cell Death Discovery (2023)
-
Astrocytes: new evidence, new models, new roles
Biophysical Reviews (2023)