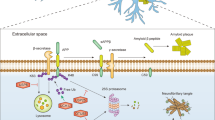
Key Points
-
Recent studies of the molecular mechanism of brain degeneration in neurodegenerative diseases have found several common features in this group of clinicopathologically different illnesses, which includes Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and transmissible spongiform encephalopathies.
-
Evidence from neuropathological and genetic studies, as well as from the generation of transgenic animal models, strongly supports the hypothesis that diverse neurodegenerative diseases are caused by the misfolding, aggregation and accumulation in the brain of an underlying protein.
-
In vitro structural studies have shown that misfolding and aggregation involve a large structural rearrangement of the protein, forming cross-β amyloid-like fibrils, which seems to be the common end point of protein aggregation in these diseases and can result in the accumulation of aggregates intra- or extracellularly. The misfolding and aggregation process depends on either hydrophobic interactions or hydrogen bonding between the protein molecules.
-
Protein misfolding and aggregation follows a seeding–nucleation mechanism modulated by several environmental factors and involving the formation of at least two intermediates: soluble oligomers and protofibrils.
-
The mechanism by which protein misfolding and aggregation is involved in neurodegeneration is unknown, but at least three general models can be proposed: loss of the physiological activity of the misfolded protein, acquisition of neurotoxicity upon protein misfolding and chronic brain inflammation triggered by the accumulation of protein deposits.
-
Although most of the data support the gain of a neurotoxic activity as the most likely mechanism of neurodegeneration, the nature of the toxic species is unknown. Recent findings indicate that soluble microaggregates, rather than large protein deposits, might be mostly implicated in neuronal damage.
-
Several strategies are being pursued to inhibit and/or reverse protein misfolding and aggregation, with the hope that some of them will result in the generation of drugs that will be useful for the treatment of neurodegenerative diseases.
-
Additional research is necessary to demonstrate definitively the involvement of protein misfolding and aggregation as a common cause of neurodegenerative diseases. Future work should also focus on understanding the contribution of alternative protein folding in other diseases and in normal cellular functioning.
Abstract
Recent evidence indicates that diverse neurodegenerative diseases might have a common cause and pathological mechanism — the misfolding, aggregation and accumulation of proteins in the brain, resulting in neuronal apoptosis. Studies from different disciplines strongly support this hypothesis and indicate that a common therapy for these devastating disorders might be possible. The aim of this article is to review the literature on the molecular mechanism of protein misfolding and aggregation, its role in neurodegeneration and the potential targets for therapeutic intervention in neurodegenerative diseases. Many questions still need to be answered and future research in this field will result in exciting new discoveries that might impact other areas of biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carrell, R. W. & Lomas, D. A. Conformational disease. Lancet 350, 134–138 (1997).
Dobson, C. M. Protein misfolding, evolution and disease. Trends Biochem. Sci. 24, 329–332 (1999).
Soto, C. Protein misfolding and disease; protein refolding and therapy. FEBS Lett. 498, 204–207 (2001).
Martin, J. B. Molecular basis of the neurodegenerative disorders. N. Eng. J. Med. 340, 1970–1980 (1999). | PubMed
Glenner, G. G. & Wong, C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 (1984). The identification of the amyloid-β protein in cerebral plaques from patients with Alzheimer's disease. This finding can be considered to be the beginning of the modern age of Alzheimer's research.
Grundke-Iqbal, I. et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 261, 6084–6089 (1986).
Forno, L. S. Neuropathology of Parkinson disease. J. Neuropathol. Exp. Neurol. 55, 259–272 (1996).
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Bruijn, L. I. et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281, 1851–1854 (1998).
Bolton, D. C., McKinley, M. P. & Prusiner, S. B. Identification of a protein that purifies with the scrapie prion. Science 218, 1309–1311 (1982). Reports the identification of the prion protein as an important constituent of the scrapie infectious agent, opening the door for the 'protein-only' hypothesis of prion propagation.
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Gutekunst, C. A. et al. Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J. Neurosci. 19, 2522–2534 (1999). | Article
McKeith, I. G. et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47, 1113–1124 (1996).
Hardy, J. & Gwinn-Hardy, K. Genetic classification of primary neurodegenerative disease. Science 282, 1075–1079 (1998).
Katzman, R. et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol. 23, 138–144 (1988).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349, 704–706 (1991).
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997). This article was the first indication of the association of α–synuclein with the pathogenesis of Parkinson's disease.
The Huntington's Disease Collaborative Research Group (HDCRG). A novel gene containing a trinucleotide repeat that is unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Hsiao, K. et al. Linkage of a prion protein missense variant to Gerstmann–Straussler syndrome. Nature 338, 342–345 (1989). The first demonstration that a protein undergoing misfolding was genetically associated with an inherited neurodegenerative disease.
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527 (1995).
Masliah, E. et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 (2000).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994). Describes the generation of an animal model of amyotropic lateral sclerosis by overexpression of mutated human superoxide dismutase 1 (SOD1). Pathological features arose despite the significant elevation of SOD1 activity, providing the first indication that the disease is not due to a decrease in SOD1 function.
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996). This paper demonstrates that the expansion of polyglutamine sequences in huntingtin leads to the pathological features of Huntington's disease (HD).
Davies, S. W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997).
Hsiao, K. K. et al. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science 250, 1587–1590 (1990).
Moechars, D. et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 274, 6483–6492 (1999). | Article |
Klement, I. A. et al. Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95, 41–53 (1998).
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Kane, M. D. et al. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci. 20, 3606–3611 (2000). | Article
Lundmark, K. et al. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc. Natl Acad. Sci. USA 99, 6979–6984 (2002). The authors reported that minute quantities of amyloid-A fibrils, given intravenously or orally, accelerated amyloid deposition in vivo , and provided evidence to indicate that amyloid-related disorders might have an infectious origin, similar to that of transmissible spongiform encephalopathy.
Xing, Y. et al. Transmission of mouse senile amyloidosis. Lab. Invest. 81, 493–499 (2001). | Article
Wickner, R. B. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264, 566–569 (1994). In this seminal paper, Wickner proposed the existence of protein-based inheritance in yeast following a prion-like mechanism to explain perplexing information about the non-Mendelian genetic elements [URE3] and [PSI]. This concept has expanded the prion phenomenon of propagation of information through changes in protein conformation.
Uptain, S. M. L. & Lindquist, S. Prions as protein-based genetic elements. Annu. Rev. Microbiol. 56, 703–741 (2002).
Cohen, A. S. & Calkins, E. Electron microscopic observation on a fibrous component in amyloid of diverse origins. Nature 183, 1202–1203 (1959). Ultrastructural study of fibrillar aggregates composed of diverse proteins, showing that despite the different amino-acid sequences of the protein components, the fibrils were strikingly similar.
Sunde, M. et al. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 273, 729–739 (1997). High-resolution structural studies by X-ray fibre diffraction of diverse fibrillar aggregates showing the β-cross conformation as a common core structure of amyloid fibrils.
Serpell, L. C., Blake, C. C. & Fraser, P. E. Molecular structure of a fibrillar Alzheimer's Aβ fragment. Biochemistry 39, 13269–13275 (2000).
Barrow, C. J., Yasuda, A., Kenny, P. T. & Zagorski, M. G. Solution conformations and aggregational properties of synthetic amyloid β-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J. Mol. Biol. 225, 1075–1093 (1992).
Pan, K. M. et al. Conversion of α-helices into β-sheet features in the formation of scrapie prion poteins. Proc. Natl Acad. Sci. USA 90, 10962–10966 (1993). | Article
Conway, K. A., Harper, J. D. & Lansbury, P. T. Fibrils formed in vitro from α-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry 39, 2552–2563 (2000).
Chen, S., Berthelier, V., Hamilton, J. B., O'Nuallain, B. & Wetzel, R. Amyloid-like features of polyglutamine aggregates and their assembly kinetics. Biochemistry 41, 7391–7399 (2002).
Serpell, L. C., Berriman, J., Jakes, R., Goedert, M. & Crowther, R. A. Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc. Natl Acad. Sci. USA 97, 4897–4902 (2000).
Sadqi, M. et al. α-Helix structure in Alzheimer's disease aggregates of tau-protein. Biochemistry 41, 7150–7155 (2002).
Teplow, D. B. Structural and kinetic features of amyloid β-protein fibrillogenesis. Amyloid 5, 121–142 (1998).
Castaño, E. M. et al. In vitro formation of amyloid fibrils from two synthetic peptides of different lengths homologous to Alzheimer's disease β-protein. Biochem. Biophys. Res. Commun. 141, 782–789 (1986). The first report of amyloid fibril formation by synthetic peptides in vitro , opening a large area of research on the biochemical and structural determinants of amyloid formation.
Hilbich, C., Kisters-Woike, B., Reed, J., Masters, C. L. & Beyreuther, K. Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer's disease βA4 peptides. J. Mol. Biol. 228, 460–473 (1992).
Jarrett, J. T., Berger, E. P. & Lansbury, P. T. Jr. The C-terminus of the β protein is critical in amyloidogenesis. Ann. NY Acad. Sci. 695, 144–148 (1993).
Soto, C., Casta–o, E. M., Frangione, B. & Inestrosa, N. C. The α-helical to β-strand transition in the amino-terminal fragment of the amyloid β-peptide modulates amyloid formation. J. Biol. Chem. 270, 3063–3067 (1995).
Wood, J. D., Wetzel, R., Martin, J. D. & Hurle, M. R. Prolines and amyloidogenicity in fragments of the Alzheimer's peptide β/A4. Biochemistry 34, 724–730 (1995).
Tagliavini, F. et al. Synthetic peptides homologous to prion protein residues 106–147 form amyloid-like fibrils in vitro. Proc. Natl Acad. Sci. USA 90, 9678–9682 (1993). | Article
Ueda, K. et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 11282–11286 (1993). | Article
Zoghbi, H. Y. & Orr, H. T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Scherzinger, E. et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90, 549–558 (1997).
Perutz, M. F., Johnson, T., Suzuki, M. & Finch, J. T. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc. Natl Acad. Sci. USA 91, 5355–5358 (1994). | Article
DePace, A. H., Santoso, A., Hillner, P. & Weissman, J. S. A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell 93, 1241–1252 (1998).
Mrak, R. E., Griffin, S. T. & Graham, D. I. Aging-associated changes in human brain. J. Neuropathol. Exp. Neurol. 56, 1269–1275 (1997).
Scherzinger, E. et al. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington's disease pathology. Proc. Natl Acad. Sci. USA 96, 4604–4609 (1999).
Wood, S. J. et al. α-Synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease. J. Biol. Chem. 274, 19509–19512 (1999).
LeVine, H. III. Soluble multimeric Alzheimer β(1-40) pre-amyloid complexes in dilute solution. Neurobiol. Aging 16, 755–764 (1995).
Kuo, Y. M. et al. Water-soluble Aβ(N-40, N-42) oligomers in normal and Alzheimer disease brains. J. Biol. Chem. 271, 4077–4081 (1996).
Lambert, M. P. et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA 95, 6448–6453 (1998).
Walsh, D. M. et al. Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Walsh, D. M., Lomakin, A., Benedek, G. B., Condron, M. M. & Teplow, D. B. Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem. 272, 22364–22372 (1997).
Walsh, D. M. et al. Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274, 25945–25952 (1999). | Article
Harper, J. D., Wong, S. S., Lieber, C. M. & Lansbury, P. T. Jr. Assembly of Aβ amyloid protofibrils: an in vitro model for a possible early event in Alzheimer's disease. Biochemistry 38, 8972–8980 (1999).
Conway, K. A., Harper, J. D. & Lansbury, P. T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nature Med. 4, 1318–1320 (1998).
Hyman, B. T. & Van Hoesen, G. W. Neuron numbers in Alzheimer's disease: cell-specific pathology. Neurobiol. Aging 8, 555–556 (1987).
Myers, R. H. et al. Decreased neuronal and increased oligodendroglial densities in Huntington's disease caudate nucleus. J. Neuropathol. Exp. Neurol. 50, 729–742 (1991).
Hughes, J. T. Pathology of amyotrophic lateral sclerosis. Adv. Neurol. 36, 61–74 (1982).
Gray, F. et al. Neuronal apoptosis in Creutzfeldt–Jakob disease. J. Neuropathol. Exp. Neurol. 58, 321–328 (1999).
Mattson, M. P. Apoptosis in neurodegenerative disorders. Nature Rev. Mol. Cell Biol. 1, 120–129 (2000).
Reaume, A. G. et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genet. 13, 43–47 (1996).
Borchelt, D. R. et al. Superoxide dismutase 1 subunits with mutations linked to familial amyotrophic lateral sclerosis do not affect wild-type subunit function. J. Biol. Chem. 270, 3234–3238 (1995).
Cattaneo, E. et al. Loss of normal huntingtin function: new developments in Huntington's disease research. Trends Neurosci. 24, 182–188 (2001).
Dragatsis, I., Levine, M. S. & Zeitlin, S. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nature Genet. 26, 300–306 (2000).
Martins, V. R. et al. Cellular prion protein: on the road for functions. FEBS Lett. 512, 25–28 (2002).
Chiarini, L. B. et al. Cellular prion protein transduces neuroprotective signals. EMBO J. 21, 3317–3326 (2002).
Kuwahara, C. et al. Prions prevent neuronal cell-line death. Nature 400, 225–226 (1999).
Kurschner, C. & Morgan, J. I. The cellular prion protein (PrP) selectively binds to Bcl-2 in the yeast two-hybrid system. Brain Res. Mol. Brain Res. 30, 165–168 (1995).
Bounhar, Y., Zhang, Y., Goodyer, C. G. & LeBlanc, A. Prion protein protects human neurons against Bax-mediated apoptosis. J. Biol. Chem. 276, 39145–39149 (2001).
Xu, X. et al. Wild-type but not Alzheimer-mutant amyloid precursor protein confers resistance against p53-mediated apoptosis. Proc. Natl Acad. Sci. USA 96, 7547–7552 (1999).
da Costa, C. A., Ancolio, K. & Checler, F. Wild-type but not Parkinson's disease-related ala-53 → Thr mutant α-synuclein protects neuronal cells from apoptotic stimuli. J. Biol. Chem. 275, 24065–24069 (2000).
Zheng, H. et al. Mice deficient for the amyloid precursor protein gene. Ann. NY Acad. Sci. 777, 421–426 (1996).
Abeliovich, A. et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 (2000).
Bueler, H. et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356, 577–582 (1992).
Behrens, A. & Aguzzi, A. Small is not beautiful: antagonizing functions for the prion protein PrPC and its homologue Dpl. Trends Neurosci. 25, 150–154 (2002).
Mallucci, G. R. et al. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 21, 202–210 (2002).
Bush, A. I. Metals and neuroscience. Curr. Opin. Chem. Biol. 4, 184–191 (2000).
Loo, D. T. et al. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc. Natl Acad. Sci. USA 90, 7951–7955 (1993). | Article
Forloni, G. et al. Neurotoxicity of a prion protein fragment. Nature 362, 543–546 (1993).
Lunkes, A. & Mandel, J. L. A cellular model that recapitulates major pathogenic steps of Huntington's disease. Hum. Mol. Genet. 7, 1355–1361 (1998).
El-Agnaf, O. M. et al. Aggregates from mutant and wild-type α-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of β-sheet and amyloid-like filaments. FEBS Lett. 440, 71–75 (1998).
Bucciantini, M. et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416, 507–511 (2002). This study shows that the misfolding and aggregation of proteins that are not connected to disease are highly cytotoxic, supporting the idea of a common mechanism of toxicity in protein conformational disorders.
Yan, S. D. et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nature Med. 6, 643–651 (2000).
Cummings, C. J. et al. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nature Genet. 19, 148–154 (1998).
Ii, K., Ito, H., Tanaka, K. & Hirano, A. Immunocytochemical co-localization of the proteasome in ubiquitinated structures in neurodegenerative diseases and the elderly. J. Neuropathol. Exp. Neurol. 56, 125–131 (1997).
Arispe, N., Rojas, E. & Pollard, H. B. Alzheimer disease amyloid-β protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc. Natl Acad. Sci. USA 90, 567–571 (1993). | Article
Lin, M. X., Mirzabekov, T. & Kagan, B. L. Channel formation by a neurotoxic prion protein fragment. J. Biol. Chem. 272, 44–47 (1997).
Behl, C., Davis, J. B., Lesley, R. & Schubert, D. Hydrogen peroxide mediates amyloid-β toxicity. Cell 77, 817–827 (1994).
Hsu, L. J. et al. α-Synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 157, 401–410 (2000). | Article
Tompkins, M. M. & Hill, W. D. Contribution of somal Lewy bodies to neuronal death. Brain Res. 775, 24–29 (1997).
Bondareff, W., Mountjoy, C. Q., Roth, M. & Hauser, D. L. Neurofibrillary degeneration and neuronal loss in Alzheimer's disease. Neurobiol. Aging 10, 709–715 (1989).
Watase, K. et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron 34, 905–919 (2002).
Saudou, F., Finkbeiner, S., Devys, D. & Greenberg, M. E. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55–66 (1998).
Hartley, D. M. et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876–8884 (1999). | Article
Goldberg, M. S. & Lansbury, P. T. Jr. Is there a cause-and-effect relationship between α-synuclein fibrillization and Parkinson's disease? Nature Cell Biol. 2, E115–E119 (2000).
Hyman, B. T., Marzloff, K. & Arriagada, P. V. The lack of accumulation of senile plaques or amyloid burden in Alzheimer's disease suggest a dynamic balance between amyloid deposition and dissolution. J. Neuropathol. Exp. Neurol. 52, 594–600 (1993).
Kim, S., Nollen, E. A., Kitagawa, K., Bindokas, V. P. & Morimoto, R. I. Polyglutamine protein aggregates are dynamic. Nature Cell Biol. 4, 826–831 (2002).
McGeer, P. L. & McGeer, E. G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Brain Res. Rev. 21, 195–218 (1995).
Wyss-Coray, T. & Mucke, L. Inflammation in neurodegenerative disease — a double-edged sword. Neuron 35, 419–432 (2002).
Itagaki, S., McGeer, P. L., Akiyama, H., Zhu, S. & Selkoe, D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 24, 173–182 (1989).
Sapp, E. et al. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 60, 161–172 (2001).
Kawamata, T., Akiyama, H., Yamada, T. & McGeer, P. L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 140, 691–707 (1992).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291 (1988).
Muhleisen, H., Gehrmann, J. & Meyermann, R. Reactive microglia in Creutzfeldt–Jakob disease. Neuropathol. Appl. Neurobiol. 21, 505–517 (1995).
Xia, M. Q. & Hyman, B. T. Chemokines/chemokine receptors in the central nervous system and Alzheimer's disease. J. Neurovirol. 5, 32–41 (1999).
Peyrin, J. M. et al. Microglial cells respond to amyloidogenic PrP peptide by the production of inflammatory cytokines. Neuroreport 10, 723–729 (1999).
Yates, S. L. et al. Amyloid-β and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J. Neurochem. 74, 1017–1025 (2000).
McGeer, P. L., Schulzer, M. & McGeer, E. G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 47, 425–432 (1996).
Wyss-Coray, T. Y. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc. Natl Acad. Sci. USA 99, 10837–10842 (2002).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999). Describes pioneer work on the immunization approach to reduce amyloid deposition in Alzheimer's disease.
Morgan, D. et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408, 982–985 (2000).
Janus, C. et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408, 979–982 (2000).
Schenk, D. Amyloid-β immunotherapy for Alzheimer's disease: the end of the beginning. Nature Rev. Neurosci. 3, 824–828 (2002).
Tatzelt, J., Prusiner, S. B. & Welch, W. J. Chemical chaperones interfere with the formation of scrapie prion protein. EMBO J. 15, 6363–6373 (1996).
Salomon, A. R., Marcinowski, K. J., Friedland, R. & Zagorski, M. G. Nicotine inhibits amyloid formation by the β-peptide. Biochemistry 35, 13568–13578 (1996).
Miroy, G. J. et al. Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proc. Natl Acad. Sci. USA 93, 15051–15056 (1996).
Villegas, V. et al. Protein engineering as a strategy to avoid formation of amyloid fibrils. Protein Sci. 9, 1700–1708 (2000).
Coelho, T. et al. Compound heterozygotes of transthyretin Met30 and transthyretin Met119 are protected from the devastating effects of familial amyloid polyneuropathy. Neuromusc. Disord. 6, S20 (1996).
Alves, I. L., Hays, M. T. & Saraiva, M. J. Comparative stability and clearance of Met30 and Met119 transthyretin. Eur. J. Biochem. 249, 662–668 (1996).
Hammarstrom, P., Schneider, F. & Kelly, J. W. Trans-suppression of misfolding in an amyloid disease. Science 293, 2459–2462 (2001). This manuscript describes the concept of creating over-stabilized proteins to prevent protein misfolding and aggregation.
Perrier, V. et al. Dominant-negative inhibition of prion replication in transgenic mice. Proc. Natl Acad. Sci. USA 99, 13079–13084 (2002).
Soto, C. et al. β-Sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer's therapy. Nature Med. 4, 822–826 (1998). Reports the use of β-sheet breaker peptides for the treatment of protein misfolding disorders.
Permanne, B. et al. Reduction of amyloid load and cerebral damage in a transgenic animal model of Alzheimer's disease by treatment with a β-sheet breaker peptide. FASEB J. 16, 860–862 (2002).
Soto, C. et al. Reversion of prion protein conformational changes by synthetic β-sheet breaker peptides. Lancet 355, 192–197 (2000).
Merlini, G. et al. Interaction of the anthracycline 4′-iodo-4′-deoxydoxorubicin with amyloid fibrils: inhibition of fibrillogenesis. Proc. Natl Acad. Sci. USA 92, 2959–2964 (1995). | Article
Forloni, G., Colombo, L., Girola, L., Tagliavini, F. & Salmona, M. Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett. 487, 404–407 (2001).
Aguzzi, A., Glatzel, M., Montrasio, F., Prinz, M. & Heppner, F. L. Interventional strategies against prion diseases. Nature Rev. Neurosci. 2, 745–749 (2001).
Sacchettini, J. C. & Kelly, J. W. Therapeutic strategies for human amyloid diseases. Nature Rev. Drug Discov. 1, 267–275 (2002).
LeVine, H. The challenge of inhibiting Aβ polymerization. Curr. Med. Chem. 9, 1121–1133 (2002).
Yamamoto, A., Lucas, J. J. & Hen, R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell 101, 57–66 (2000). Experiments with an inducible transgenic model of HD showed that eliminating the continued production of mutant huntingtin resulted in the disappearance of nuclear deposits, indicating that normal clearance mechanisms can remove aggregated materials.
Sigurdsson, E. M. et al. Immunization delays the onset of prion disease in mice. Am. J. Pathol. 161, 13–17 (2002).
Pepys, M. B. et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 417, 254–259 (2002).
Kisilevsky, R. et al. Arresting amyloidosis in vivo using small-molecule anionic sulphonates or sulphates: implications for Alzheimer's disease. Nature Med. 1, 143–148 (1995).
Cherny, R. A. et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676 (2001).
Fandrich, M., Fletcher, M. A. & Dobson, C. M. Amyloid fibrils from muscle myoglobin. Nature 410, 165–166 (2001). This article shows that even myoglobin, the prototype of an α-helical and globular protein, can form amyloid fibrils under appropriate conditions.
Pertinhez, T. A. et al. Amyloid fibril formation by a helical cytochrome. FEBS Lett. 495, 184–186 (2001).
Acknowledgements
I thank C. Adessi, K. Maundrell, Y. Fezoui and C. Hetz for stimulating discussions of the ideas described in this review. I also appreciate the work of K. Maundrell and J. Delamarter in critically reading and correcting this manuscript and the continuous support of S. Fumero.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
OMIM
Swiss-Prot
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- CHAPERONE PROTEINS
-
A family of cellular proteins that mediate the correct folding of other polypeptides, and in some cases their assembly into oligomeric structures, but which are not components of those final structures. It is believed that chaperone proteins assist polypeptides in folding by inhibiting alternative assembly pathways that produce nonfunctional structures.
- PROTEIN FOLDING
-
The process by which a protein acquires its native tridimensional structure. Under physiological conditions, each protein has a unique stable folded structure, but in conformational disorders the polypeptide chain adopts an alternative structure, associated with the pathogenesis of the disease.
- PROTEIN CONFORMATIONAL DISORDERS
-
A recently recognized group of diseases in which the key event is the misfolding, aggregation and tissue deposition of a protein.
- AMYLOID
-
A general term for a variety of protein aggregates that accumulate as extracellular fibrils of 7–10 nm and have common structural features, including a β-pleated sheet conformation and the ability to bind such dyes as Congo red and thioflavins S and T.
- ASTROGLIOSIS
-
Proliferation and ramification of glial cells in response to brain damage.
- SPONGIFORM DEGENERATION
-
The brain damage associated with TSE or prion diseases, consisting of extensive vacuolization of neuronal cells.
- β-SHEETS
-
β-Sheets and α-helices are the two types of prevalent, repetitive secondary structure in folded proteins. β-Sheets are formed of alternating peptide pleated strands linked by hydrogen bonding between the NH and CO groups of the peptide bond. Formation of β-sheets can be stabilized by protein oligomerization or aggregation.
- β-SHEET OLIGOMERS
-
Structures containing several units of a protein organized in a β-pleated sheet conformation.
- APOPTOSIS
-
The process of programmed cell death, characterized by distinctive morphological changes in the nucleus and cytoplasm, chromatin cleavage at regularly spaced sites, and the endonucleolytic cleavage of genomic DNA.
- CASPASES
-
A family of intracellular cysteine endopeptidases that have a key role in inflammation and mammalian apoptosis. They cleave proteins at specific aspartate residues.
- OXIDATIVE STRESS
-
A disturbance in the pro-oxidant–antioxidant balance in favour of the former, leading to potential cellular damage. Indicators of oxidative stress include damaged DNA bases, protein oxidation and lipid peroxidation products.
- MENINGOENCEPHALITIS
-
An inflammatory process involving the brain and meninges, most often produced by pathogenic organisms that invade the central nervous system, and occasionally by toxins, autoimmune disorders and other conditions.
Rights and permissions
About this article
Cite this article
Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4, 49–60 (2003). https://doi.org/10.1038/nrn1007
Issue Date:
DOI: https://doi.org/10.1038/nrn1007
This article is cited by
-
Brain-targeted delivery of neuroprotective survival gene minimizing hematopoietic cell contamination: implications for Parkinson’s disease treatment
Journal of Translational Medicine (2024)
-
Simultaneous binding characterization of different chromium speciation to serum albumin
BioMetals (2024)
-
Liquid–liquid phase separation in Alzheimer’s disease
Journal of Molecular Medicine (2024)
-
α-Synuclein conformers reveal link to clinical heterogeneity of α-synucleinopathies
Translational Neurodegeneration (2023)
-
Intra-hippocampal cis-P tau microinjection induces long-term changes in behavior and synaptic plasticity in mice
Behavioral and Brain Functions (2023)