Abstract
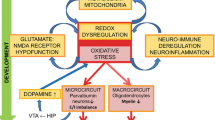
Molecular, genetic and pathological evidence suggests that deficits in GABAergic parvalbumin-positive interneurons contribute to schizophrenia pathophysiology through alterations in the brain's excitation–inhibition balance that result in impaired behaviour and cognition. Although the factors that trigger these deficits are diverse, there is increasing evidence that they converge on a common pathological hub that involves NMDA receptor hypofunction and oxidative stress. These factors have been separately linked to schizophrenia pathogenesis, but evidence now suggests that they are mechanistically interdependent and contribute to a common schizophrenia-associated pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brown, A. S. The environment and susceptibility to schizophrenia. Prog. Neurobiol. 93, 23–58 (2011).
Lewis, D. A., Curley, A. A., Glausier, J. R. & Volk, D. W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67 (2012).
Insel, T. R. Rethinking schizophrenia. Nature 468, 187–193 (2010).
Mighdoll, M. I., Tao, R., Kleinman, J. E. & Hyde, T. M. Myelin, myelin-related disorders, and psychosis. Schizophr. Res. 161, 85–93 (2015).
Uhlhaas, P. J. & Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100–113 (2010).
Paoletti, P., Bellone, C. & Zhou, Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400 (2013).
Wyllie, D. J., Livesey, M. R. & Hardingham, G. E. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 74, 4–17 (2013).
Bell, K. F. & Hardingham, G. E. The influence of synaptic activity on neuronal health. Curr. Opin. Neurobiol. 21, 299–305 (2011).
Javitt, D. C. & Zukin, S. R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 148, 1301–1308 (1991).
Coyle, J. T., Basu, A., Benneyworth, M., Balu, D. & Konopaske, G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb. Exp. Pharmacol. 213, 267–295 (2012).
Luby, E. D., Cohen, B. D., Rosenbaum, G., Gottlieb, J. S. & Kelley, R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch. Neurol. Psychiatry 81, 363–369 (1959).
Krystal, J. H. et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 51, 199–214 (1994).
Anis, N. A., Berry, S. C., Burton, N. R. & Lodge, D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br. J. Pharmacol. 79, 565–575 (1983).
Dalmau, J. et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 7, 1091–1098 (2008).
Javitt, D. C. et al. Translating glutamate: from pathophysiology to treatment. Sci. Transl Med. 3, 102mr2 (2011).
Schwarcz, R. et al. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry 50, 521–530 (2001).
Hashimoto, K. et al. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 767–769 (2005).
Weickert, C. S. et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatry 18, 1185–1192 (2013).
Pilowsky, L. S. et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol. Psychiatry 11, 118–119 (2006).
Howes, O., McCutcheon, R. & Stone, J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J. Psychopharmacol. 29, 97–115 (2015).
Group, S. W. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Balu, D. T. & Coyle, J. T. The NMDA receptor 'glycine modulatory site' in schizophrenia: D-serine, glycine, and beyond. Curr. Opin. Pharmacol. 20, 109–115 (2015).
Tarabeux, J. et al. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl Psychiatry 1, e55 (2011).
Myers, R. A. et al. A population genetic approach to mapping neurological disorder genes using deep resequencing. PLoS Genet. 7, e1001318 (2011).
Carlsson, M. & Carlsson, A. The NMDA antagonist MK-801 causes marked locomotor stimulation in monoamine-depleted mice. J. Neural Transm. 75, 221–226 (1989).
Morris, R., Anderson, E., Lynch, G. & Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an NMDA receptor antagonist, AP5. Nature 319, 774–776 (1986).
Mohn, A. R., Gainetdinov, R. R., Caron, M. G. & Koller, B. H. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98, 427–436 (1999).
Balu, D. T. et al. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc. Natl Acad. Sci. USA 110, E2400–E2409 (2013).
Stefani, M. R. & Moghaddam, B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol. Psychiatry 57, 433–436 (2005).
Ikonomidou, C. et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283, 70–74 (1999).
Wang, X., Pinto-Duarte, A., Sejnowski, T. J. & Behrens, M. M. How Nox2-containing NADPH oxidase affects cortical circuits in the NMDA receptor antagonist model of schizophrenia. Antioxid. Redox Signal. 18, 1444–1462 (2013).
Belforte, J. E. et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci. 13, 76–83 (2010).
Korotkova, T., Fuchs, E. C., Ponomarenko, A., von Engelhardt, J. & Monyer, H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557–569 (2010).
Dringen, R., Pawlowski, P. G. & Hirrlinger, J. Peroxide detoxification by brain cells. J. Neurosci. Res. 79, 157–165 (2005).
Hardingham, G. E. & Lipton, S. A. Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid. Redox Signal. 14, 1421–1424 (2011).
Fernandez-Fernandez, S., Almeida, A. & Bolanos, J. P. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem. J. 443, 3–11 (2012).
Kennedy, K. A., Sandiford, S. D., Skerjanc, I. S. & Li, S. S. Reactive oxygen species and the neuronal fate. Cell. Mol. Life Sci. 69, 215–221 (2012).
Yao, J. K. & Keshavan, M. S. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid. Redox Signal. 15, 2011–2035 (2011).
Do, K. Q., Cabungcal, J. H., Frank, A., Steullet, P. & Cuenod, M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 19, 220–230 (2009).
Flatow, J., Buckley, P. & Miller, B. J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry 74, 400–409 (2013).
Coughlin, J. M. et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol. Psychiatry 18, 10–11 (2013).
Martins-de-Souza, D., Harris, L. W., Guest, P. C. & Bahn, S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid. Redox Signal. 15, 2067–2079 (2011).
Do, K. Q. et al. Schizophrenia: glutathione deficit in cerebro spinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 12, 3721–3728 (2000).
Gawryluk, J. W., Wang, J. F., Andreazza, A. C., Shao, L. & Young, L. T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 14, 123–130 (2011).
Matsuzawa, D. & Hashimoto, K. Magnetic resonance spectroscopy study of the antioxidant defense system in schizophrenia. Antioxid. Redox Signal. 15, 2057–2065 (2011).
Gysin, R. et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc. Natl Acad. Sci. USA 104, 16621–16626 (2007).
Rodriguez-Santiago, B. et al. Association of common copy number variants at the glutathione S-transferase genes and rare novel genomic changes with schizophrenia. Mol. Psychiatry 15, 1023–1033 (2010).
Steullet, P. et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 30, 2547–2558 (2010).
Kulak, A., Cuenod, M. & Do, K. Q. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav. Brain Res. 226, 563–570 (2012).
Jacobsen, J. P., Rodriguiz, R. M., Mork, A. & Wetsel, W. C. Monoaminergic dysregulation in glutathione-deficient mice: possible relevance to schizophrenia? Neuroscience 132, 1055–1072 (2005).
Cabungcal, J. H. et al. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: relevance to schizophrenia. Neurobiol. Dis. 26, 634–645 (2007).
Gokhale, A. et al. Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1. J. Neurosci. 32, 3697–3711 (2012).
Yap, M. Y., Lo, Y. L., Talbot, K. & Ong, W. Y. Oxidative stress reduces levels of dysbindin-1A via its PEST domain. Neurochem. Int. 79, 65–69 (2014).
Natarajan, S. K. et al. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic. Biol. Med. 53, 1181–1191 (2012).
Goldsmit, Y., Erlich, S. & Pinkas-Kramarski, R. Neuregulin induces sustained reactive oxygen species generation to mediate neuronal differentiation. Cell. Mol. Neurobiol. 21, 753–769 (2001).
Filiou, M. D., Teplytska, L., Otte, D. M., Zimmer, A. & Turck, C. W. Myelination and oxidative stress alterations in the cerebellum of the G72/G30 transgenic schizophrenia mouse model. J. Psychiatr. Res. 46, 1359–1365 (2012).
Johnson, A. W. et al. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc. Natl Acad. Sci. USA 110, 12462–12467 (2013).
Cabungcal, J. H. et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 83, 1073–1084 (2014).
Otte, D. M. et al. N-acetyl cysteine treatment rescues cognitive deficits induced by mitochondrial dysfunction in G72/G30 transgenic mice. Neuropsychopharmacology 36, 2233–2243 (2011).
Wirth, E. K. et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 24, 844–852 (2010).
Cabungcal, J. H., Steullet, P., Kraftsik, R., Cuenod, M. & Do, K. Q. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol. Psychiatry 73, 574–582 (2013).
Cabungcal, J. H. et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl Acad. Sci. USA 110, 9130–9135 (2013).
Morishita, H., Cabungcal, J. H., Chen, Y., Do, K. Q. & Hensch, T. K. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol. Psychiatry 78, 396–402 (2015).
Greenhill, S. D. et al. Adult cortical plasticity depends on an early postnatal critical period. Science 349, 424–427 (2015).
Hikida, T. et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc. Natl Acad. Sci. USA 104, 14501–14506 (2007).
Schiavone, S. et al. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol. Psychiatry 66, 384–392 (2009).
Back, S. A. & Rosenberg, P. A. Pathophysiology of glia in perinatal white matter injury. Glia 62, 1790–1815 (2014).
Monin, A. et al. Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. Mol. Psychiatry 20, 827–838 (2015).
Lipton, S. A. et al. Cysteine regulation of protein function — as exemplified by NMDA-receptor modulation. Trends Neurosci. 25, 474–480 (2002).
Choi, Y. B. & Lipton, S. A. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron 23, 171–180 (1999).
Kohr, G., Eckardt, S., Luddens, H., Monyer, H. & Seeburg, P. H. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron 12, 1031–1040 (1994).
Steullet, P., Neijt, H. C., Cuenod, M. & Do, K. Q. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience 137, 807–819 (2006).
Guidi, M., Kumar, A. & Foster, T. C. Impaired attention and synaptic senescence of the prefrontal cortex involves redox regulation of NMDA receptors. J. Neurosci. 35, 3966–3977 (2015).
Bodhinathan, K., Kumar, A. & Foster, T. C. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. 30, 1914–1924 (2010).
Papadia, S. et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 11, 476–487 (2008).
Radonjic, N. V. et al. Decreased glutathione levels and altered antioxidant defense in an animal model of schizophrenia: long-term effects of perinatal phencyclidine administration. Neuropharmacology 58, 739–745 (2010).
Bell, K. F. & Hardingham, G. E. CNS peroxiredoxins and their regulation in health and disease. Antioxid. Redox Signal. 14, 1467–1477 (2011).
Hardingham, G. E. & Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696 (2010).
Baxter, P. S. et al. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat. Commun. 6, 6761 (2015).
Jiang, Z., Rompala, G. R., Zhang, S., Cowell, R. M. & Nakazawa, K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol. Psychiatry 73, 1024–1034 (2013).
Moghaddam, B. & Krystal, J. H. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr. Bull. 38, 942–949 (2012).
Powell, S. B., Sejnowski, T. J. & Behrens, M. M. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology 62, 1322–1331 (2012).
Bell, K. F. et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat. Commun. 6, 7066 (2015).
Gan, L. & Johnson, J. A. Oxidative damage and the Nrf2–ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta 1842, 1208–1218 (2014).
Jimenez-Blasco, D., Santofimia-Castano, P., Gonzalez, A., Almeida, A. & Bolanos, J. P. Astrocyte NMDA receptors' activity sustains neuronal survival through a Cdk5–Nrf2 pathway. Cell Death Differ. 22, 1877–1889 (2015).
Bell, K. F. et al. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc. Natl Acad. Sci. USA 108, E1–E2; author reply E3–E4 (2011).
Bell, K. F., Fowler, J. H., Al-Mubarak, B., Horsburgh, K. & Hardingham, G. E. Activation of Nrf2-regulated glutathione pathway genes by ischemic preconditioning. Oxid. Med. Cell Longev. 2011, 689524 (2011).
Deighton, R. F. et al. Nrf2 target genes can be controlled by neuronal activity in the absence of Nrf2 and astrocytes. Proc. Natl Acad. Sci. USA 111, E1818–E1820 (2014).
Lewerenz, J. et al. Phosphoinositide 3-kinases upregulate system xc− via eukaryotic initiation factor 2α and activating transcription factor 4 — a pathway active in glioblastomas and epilepsy. Antioxid. Redox Signal. 20, 2907–2922 (2014).
Reus, G. Z. et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300, 141–154 (2015).
Kneeland, R. E. & Fatemi, S. H. Viral infection, inflammation and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 42, 35–48 (2013).
Giovanoli, S. et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339, 1095–1099 (2013).
Ibi, D. et al. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav. Brain Res. 206, 32–37 (2010).
Beloosesky, R., Gayle, D. A. & Ross, M. G. Maternal N-acetylcysteine suppresses fetal inflammatory cytokine responses to maternal lipopolysaccharide. Am. J. Obstet. Gynecol. 195, 1053–1057 (2006).
Lante, F. et al. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus 18, 602–609 (2008).
Buelna-Chontal, M. & Zazueta, C. Redox activation of Nrf2 and NF-κB: a double end sword? Cell. Signal. 25, 2548–2557 (2013).
Jin, W. et al. Disruption of Nrf2 enhances upregulation of nuclear factor-κB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm. 2008, 725174 (2008).
Pan, H., Wang, H., Wang, X., Zhu, L. & Mao, L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm. 2012, 217580 (2012).
Liu, G. H., Qu, J. & Shen, X. NF-κB/p65 antagonizes Nrf2–ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 1783, 713–727 (2008).
Kantrowitz, J. et al. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry 2, 403–412 (2015).
Li, Y. et al. Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nat. Commun. 4, 1760 (2013).
Lavoie, S. et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 33, 2187–2199 (2008).
Berk, M. et al. N-acetyl cysteine as a glutathione precursor for schizophrenia — a double-blind, randomized, placebo-controlled trial. Biol. Psychiatry 64, 361–368 (2008).
Carmeli, C., Knyazeva, M. G., Cuenod, M. & Do, K. Q. Glutathione precursor N-acetyl-cysteine modulates EEG synchronization in schizophrenia patients: a double-blind, randomized, placebo-controlled trial. PLoS ONE 7, e29341 (2012).
Farokhnia, M. et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin. Neuropharmacol. 36, 185–192 (2013).
Farr, S. A. et al. The antioxidants α-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem. 84, 1173–1183 (2003).
Amminger, G. P., Schafer, M. R., Schlogelhofer, M., Klier, C. M. & McGorry, P. D. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat. Commun. 6, 7934 (2015).
Shiina, A. et al. An open study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin. Psychopharmacol. Neurosci. 13, 62–67 (2015).
Fox, R. J. et al. BG-12 (dimethyl fumarate): a review of mechanism of action, efficacy, and safety. Curr. Med. Res. Opin. 30, 251–262 (2014).
Gupta, K. et al. Human embryonic stem cell derived astrocytes mediate non-cell-autonomous neuroprotection through endogenous and drug-induced mechanisms. Cell Death Differ. 19, 779–787 (2012).
Burnashev, N. & Szepetowski, P. NMDA receptor subunit mutations in neurodevelopmental disorders. Curr. Opin. Pharmacol. 20, 73–82 (2015).
O'Roak, B. J. et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622 (2012).
Lemke, J. R. et al. GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy. Ann. Neurol. 75, 147–154 (2014).
Lemke, J. R. et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat. Genet. 45, 1067–1072 (2013).
Freunscht, I. et al. Behavioral phenotype in five individuals with de novo mutations within the GRIN2B gene. Behav. Brain Funct. 9, 20 (2013).
Endele, S. et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 42, 1021–1026 (2010).
Lesca, G. et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat. Genet. 45, 1061–1066 (2013).
Carvill, G. L. et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat. Genet. 45, 1073–1076 (2013).
Acknowledgements
The K.Q.D. laboratory is supported by the Swiss National Science Foundation (grants # 31–116689, # 310030_135736/1, #320030_122419), the National Center of Competence in Research (NCCR) SYNAPSY (The Synaptic Bases of Mental Diseases; grant # 51AU40_125759), the Avina Foundation, the Damm-Etienne Foundation and the Alamaya Foundation (to K.Q.D.). The G.E.H. laboratory is supported by the UK Medical Research Council, the Wellcome Trust, and a Biogen Idec/University of Edinburgh Joint Discovery Research Collaboration. The authors thank K. Marwick for preparing Figure 1. The authors also thank M. Cuenod, P. Steullet, K. Marwick and D. Wyllie for their helpful comments on the article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hardingham, G., Do, K. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci 17, 125–134 (2016). https://doi.org/10.1038/nrn.2015.19
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2015.19
This article is cited by
-
Perturbed iron biology in the prefrontal cortex of people with schizophrenia
Molecular Psychiatry (2023)
-
Low protein-induced intrauterine growth restriction as a risk factor for schizophrenia phenotype in a rat model: assessing the role of oxidative stress and neuroinflammation interaction
Translational Psychiatry (2023)
-
Characterization of early psychosis patients carrying a genetic vulnerability to redox dysregulation: a computational analysis of mechanism-based gene expression profile in fibroblasts
Molecular Psychiatry (2023)
-
Magnetic Resonance Spectroscopy Studies of Brain Energy Metabolism in Schizophrenia: Progression from Prodrome to Chronic Psychosis
Current Psychiatry Reports (2023)
-
Lipopolysaccharide Exacerbates Ketamine-Induced Psychotic-Like Behavior, Oxidative Stress, and Neuroinflammation in Mice: Ameliorative Effect of Diosmin
Journal of Molecular Neuroscience (2023)