Abstract
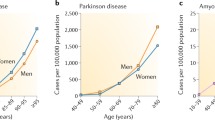
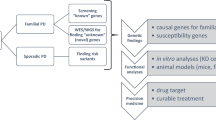
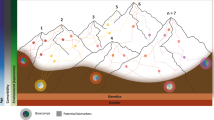
Ageing is the greatest risk factor for the development of Parkinson's disease. However, the current dogma holds that cellular mechanisms that are associated with ageing of midbrain dopamine neurons and those that are related to dopamine neuron degeneration in Parkinson's disease are unrelated. We propose, based on evidence from studies of non-human primates, that normal ageing and the degeneration of dopamine neurons in Parkinson's disease are linked by the same cellular mechanisms and, therefore, that markers of cellular risk factors accumulate with age in a pattern that mimics the pattern of degeneration observed in Parkinson's disease. We contend that ageing induces a pre-parkinsonian state, and that the cellular mechanisms of dopamine neuron demise during normal ageing are accelerated or exaggerated in Parkinson's disease through a combination of genetic and environmental factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bennett, D. A. et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N. Engl. J. Med. 334, 71–76 (1996).
Morens, D. M. et al. Epidemiologic observations on Parkinson's disease: incidence and mortality in a prospective study of middle aged men. Neurology 46, 1044–1050 (1996).
Tanner, C. M. & Goldman, S. M. Epidemiology of Parkinson's disease. Neurol. Clin. 14, 317–335 (1996).
Fearnley, J. M. & Lees, A. J. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 114, 2283–2301 (1991).
Gibb, W. R. & Lees, A. J. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J. Neurol. Neurosurg. Psychiatr. 54, 388–396 (1991).
Hornykiewicz, O. Ageing and neurotoxins as causative factors in idiopathic Parkinson's disease – a critical analysis of the neurochemical evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 13, 319–328 (1989).
Kish, S. J., Shannak, K., Rajput, A., Deck, J. H. & Hornykiewicz, O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson's disease. J. Neurochem. 58, 642–648 (1992).
Damier, P., Hirsch, E. C., Agid, Y. & Graybiel, A. M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122, 1437–1448 (1999).
Chiueh, C. C., Burns, R. S., Markey, S. P., Jacobowitz, D. M. & Kopin, I. J. Primate model of parkinsonism: selective lesion of nigrostriatal neurons by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine produces an extrapyramidal syndrome in rhesus monkeys. Life Sci. 36, 213–218 (1985).
German, D. C., Dubach, M., Askari, S., Speciale, S. G. & Bowden, D. M. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience 24, 161–174 (1988).
Kitt, C. A., Cork, L. C., Eidelberg, F., Joh, T. H. & Price, D. L. Injury of nigral neurons exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: a tyrosine hydroxylase immuunocytochemical study in monkey. Neuroscience 17, 1089–1103 (1986).
Schnieder, J. S., Yuwiler, A. & Markham, C. H. Selective loss of subpopulations of ventral mesencephalic dopaminergic neurons in the monkey following exposure to MPTP. Brain Res. 411, 144–150 (1987).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neurosci. 3, 1301–1306 (2000).
German, D. C. et al. The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration 5, 299–312 (1996).
Grant, R. J. & Clarke, P. B. Susceptibility of ascending dopamine projections to 6-hydroxydopamine in rats: effect of hypothermia. Neuroscience 115, 1281–1294 (2002).
Emborg, M. E. et al. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J. Comp. Neurol. 401, 253–265 (1998).
Zhang, Z. et al. Motor slowing and parkinsonian signs in aging rhesus monkeys mirror human aging. J. Gerontol. A Biol. Sci. Med. Sci. 55, B473–B480 (2000).
Chu, Y. & Kordower, J. H. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson's disease? Neurobiol. Dis. 25, 134–149 (2007).
Kanaan, N. M., Kordower, J. H. & Collier, T. J. Age-related accumulation of Marinesco bodies and lipofuscin in rhesus monkey midbrain dopamine neurons: relevance to selective neuronal vulnerability. J. Comp. Neurol. 502, 683–700 (2007).
Kanaan, N. M., Kordower, J. H. & Collier, T. J. Age-related changes in dopamine transporters and accumulation of 3-nitrotyrosine in rhesus monkey midbrain dopamine neurons: relevance in selective neuronal vulnerability to degeneration. Eur. J. Neurosci. 27, 3205–3215 (2008).
Kanaan, N. M., Kordower, J. H. & Collier, T. J. Age-related changes in glial cells of dopamine midbrain subregions in rhesus monkeys. Neurobiol. Aging 31, 937–952 (2008).
Kanaan, N. M., Kordower, J. H. & Collier, T. J. Age and region-specific responses of microglia, but not astrocytes, suggest a role in selective vulnerability of dopamine neurons after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure in monkeys. Glia 56, 1199–1214 (2008).
Andersen, A. H., Zhang, Z., Zhang, M., Gash, D. M. & Avison, M. J. Age-associated changes in rhesus CNS composition identified by MRI. Brain Res. 829, 90–98 (1999).
Collier, T. J. et al. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol. Dis. 26, 56–65 (2007).
Irwin, I. et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration 3, 251–265 (1994).
McCormack, A. L. et al. Aging of the nigrostriatal system in the squirrel monkey. J. Comp. Neurol. 471, 387–395 (2004).
Pakkenberg, H., Andersen, B. B., Burns, R. S. & Pakkenberg, B. A stereological study of substantia nigra in young and old rhesus monkeys. Brain Res. 693, 201–206 (1995).
Irizarry, M. C. et al. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain α-synuclein immunoreactivity. J. Neuropathol. Exp. Neurol. 57, 334–337 (1998).
Spillantini, M. G. et al. α-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Ross, O. A. et al. Genomic investigation of α-synuclein multiplication and parkinsonism. Ann. Neurol. 63, 743–750 (2008).
Jellinger, K. A. Lewy body-related α-synucleinopathy in the aged human brain. J. Neural Transm. 111, 1219–1235 (2004).
Li, W. et al. Stabilization of α-synuclein protein with ageing and familial Parkinson's disease-linked A53T mutation. J. Neurosci. 24, 7400–7409 (2004).
Maingay, M., Romero-Ramos, M., Carta, M. & Kirik, D. Ventral tegmental area dopamine neurons are resistant to human mutant α-synuclein overexpression. Neurobiol. Dis. 23, 522–532 (2006).
Shimura, H. et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nature Genet. 25, 302–305 (2000).
Cuervo, A. M., Stefanis, L., Fredenburg, R., Lansbury, P. T. & Sulzer, D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004).
Lee, H. J., Khoshaghideh, F., Patel, S. & Lee, S. J. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 24, 1888–1896 (2004).
Webb, J. L., Ravikumar, B., Atkins, J., Skepper, J. N. & Rubinsztein, D. C. α-synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 278, 25009–25013 (2003).
Chu, Y., Dodiya, H., Aebischer, P., Olanow, C. W. & Kordower, J. H. Alterations in lysosomal and proteasomal markers in Parkinson's disease: relationship to α-synuclein inclusions. Neurobiol. Dis. 35, 385–398 (2009).
Hald, A. & Lotharius, J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp. Neurol. 193, 279–290 (2005).
Jenner, P. Oxidative stress in Parkinson's disease. Ann. Neurol. 53, S26–S36 (2003).
Brunk, U. T. & Terman, A. The mitochondrial–lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur. J. Biochem. 269, 1996–2002 (2002).
Brunk, U. T. & Terman, A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 33, 611–619 (2002).
Terman, A. & Brunk, U. T. Lipofuscin: mechanisms of formation and increase with age. APMIS 106, 265–276 (1998).
Terman, A., Gustafsson, B. & Brunk, U. K. Mitochondrial damage and intralysosomal degradation in cellular aging. Mol. Aspects Med. 27, 471–482 (2006).
Siddiqi, Z. A. & Peters, A. The effect of aging on pars compacta of the substantia nigra in rhesus monkey. J. Neuropathol. Exp. Neurol. 58, 903–920 (1999).
Beach, T. G. et al. Substantia nigra Marinesco bodies are associated with decreased striatal expression of dopaminergic markers. J. Neuropathol. Exp. Neurol. 63, 329–337 (2004).
Yuen, P. & Baxter, D. W. The morphology of Marinesco bodies (paranucleolar corpuscles) in the melanin-pigmented nuclei of the brainstem. J. Neurol. Neurosurg. Psychiatr. 26, 178–183 (1963).
Strehler, B. L. On the histochemistry and ultrastructure of age pigment. Adv. Gerontol. Res. 18, 343–384 (1964).
Ulfig, N. Altered lipofuscin pigmentation in the basal nucleus (Meynert) in Parkinson's disease. Neurosci. Res. 6, 456–462 (1989).
Elsworth, J. D., Deutch, A. Y., Redmond, D. E. Jr, Sladek, J. R. Jr & Roth, R. H. Differential responsiveness to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in sub-regions of the primate substantia nigra and striatum. Life Sci. 40, 193–202 (1987).
Meredith, G. E. et al. Lysosomal malfunction accompanies α-synuclein aggregation in a progressive mouse model of Parkinson's disease. Brain Res. 956, 156–165 (2002).
Asanuma, M., Miyazaki, I., Diaz-Corrales, F. J. & Ogawa, N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis of sporadic Parkinson's disease and neurotoxin-induced parkinsonism. Acta Med. Okayama 58, 221–233 (2004).
Cantuti-Castelvetri, I., Shukitt-Hale, B. & Joseph, J. A. Dopamine neurotoxicity: age-dependent behavioral and histological effects. Neurobiol. Aging 24, 697–706 (2003).
Caudle, W. M. et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 27, 8138–8148 (2007).
Gonzalez-Hernandez, T., Barroso-Chinea, P., De La Cruz Muros, I., Del Mar Perez-Delgado, M. & Rodriguez, M. Expression of dopamine and vesicular monoamine transporters and differential vulnerability of mesostriatal dopaminergic neurons. J. Comp. Neurol. 479, 198–215 (2004).
Banati, R. B., Daniel, S. E. & Blunt, S. B. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov. Disord. 13, 221–227 (1998).
Mirza, B., Hadberg, H., Thomsen, P. & Moos, T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience 95, 425–432 (2000).
Imamura, K. et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 106, 518–526 (2003).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291 (1988).
Mogi, M. et al. Caspase activites and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J. Neural Transm. 107, 335–341 (2000).
Castano, A., Herrera, A. J., Cano, J. & Machado, A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J. Neurochem. 70, 1584–1592 (1998).
Wu, D. C. et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J. Neurosci. 22, 1763–1771 (2002).
Alladi, P. A. et al. Absence of age-related changes in nigral dopaminergic neurons of Asian Indians: relevance to lower incidence of Parkinson's disease. Neuroscience 159, 236–245 (2009).
Chu, Y., Kompliti, K., Cochran, E. J., Mufson, E. J. & Kordower, J. H. Age-related decrease in Nurr1 immunoreactivity in the human substantia nigra. J. Comp. Neurol. 450, 203–214 (2002).
Kubis, N. et al. Preservation of midbrain catecholaminergic neurons in very old human subjects. Brain 123, 366–373 (2000).
Gerhardt, G. A., Cass, W. A., Yi, A., Zhang, Z. & Gash, D. M. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J. Neurochem. 80, 168–177 (2002).
Chan, C. S., Gertler, T. S. & Surmeier, D. J. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 32, 249–256 (2009).
Liang, C. L., Sinton, C. M., Sonsalla, P. K. & German, D. C. Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration 5, 313–318 (1996).
Mosharov, E. V. et al. Interplay between cytosolic dopamine, calcium, and α-synuclein causes selective death of substantia nigra neurons. Neuron 62, 218–229 (2009).
Bender, A. et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson's disease. Nature Genet. 38, 515–517 (2006).
Kraytsberg, Y. et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nature Genet. 38, 518–520 (2006).
Morfini, G. A. et al. Axonal transport defects in neurodegenerative diseases. J. Neurosci. 29, 12776–12786 (2009).
Ren, Y., Liu, W., Jiang, H., Jiang, Q. & Feng, J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J. Biol. Chem. 280, 34105–34112 (2005).
Chauhan, N. B., Siegel, G. J. & Lee, J. M. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson's disease brain. J. Chem. Neuroanat. 21, 277–288 (2001).
Howells, D. W. et al. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp. Neurol. 166, 127–135 (2000).
Parain, K. et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson's disease substantia nigra. Neuroreport 10, 557–561 (1999).
Chung, C. Y. et al. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum. Mol. Genet. 14, 1709–1725 (2005).
Greene, J. G., Dingledine, R. & Greenamyre, J. T. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol. Dis. 18, 19–31 (2005).
Ji, K.-A. et al. Differential neutrophil infiltration contributes to regional differences in brain inflammation in the substantia nigra pars compacta and cortex. Glia 56, 1039–1047 (2008).
Lewers, J. C. et al. Consequences of impaired purine recycling in dopaminergic neurons. Neuroscience 152, 761–772 (2008).
Liss, B. et al. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nature Neurosci. 8, 1742–1751 (2005).
Nafia, I. et al. Preferential vulnerability of mesencephalic dopamine neurons to glutamate transporter dysfunction. J. Neurochem. 105, 484–496 (2008).
Villar-Cheda, B. et al. Nigral and striatal expression of angiotensin receptor expression by dopamine and angiotensin in rodents: implications for progression of Parkinson's disease. Eur. J. Neurosci. 32, 1695–1706 (2010).
Wang, H.-L. & Morales, M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J. Comp. Neurol. 509, 302–318 (2008).
Coleman, P., Finch, C. & Joseph, J. The need for multiple time points in aging studies. Neurobiol. Aging 25, 3–4 (1994).
Eshius, S. A. & Leenders, K. L. Parkinson's Disease: Symptoms and Age Dependency in Functional Neurobiology of Aging (eds Hof, P. R. & Mobbs, C. V.) 675–688 (Academic Press, San Diego, 2001).
Diedrich, N. J. H., Moore, C. G., Leurgans, S. E., Chmura, T. A. & Goetz, C. G. Parkinson disease with old-age onset: a comparative study with subjects with middle-age onset. Arch. Neurol. 60, 529–533 (2003).
Hely, M. A. et al. Age at onset: the major determinant of outcome in Parkinson's disease. Acta Neurol. Scand. 92, 455–463 (1995).
Jankovic, J. & Kapadia, A. S. Functional decline in Parkinson's disease. Arch. Neurol. 58, 1611–1615 (2001).
Muller, W. E. & Pedigo, N. W. Jr. Brain aging: a risk factor of neurodegenerative disorders and a target for therapeutic intervention. Life Sci. 55, 1975–1976 (1994).
Thal, D. R., Del Tredici, K. & Braak, H. Neurodegeneration in normal brain aging and disease. Sci. Aging Knowl. Environ. 2004, pe26 (2004).
Calne, D. B. & Langston, J. W. Aetiology of Parkinson's disease. Lancet 2, 1457–1479 (1983).
Carvey, P. M., Punati, A. & Newman, M. B. Progressive dopamine neuron loss in Parkinson's disease: the multiple hit hypothesis. Cell Transplant. 15, 239–250 (2006).
Sulzer, D. Multiple hit hypothesis for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 30, 244–250 (2007).
Chu, Y. et al. Nurr1 in Parkinson's disease and related disorders. J. Comp. Neurol. 494, 495–514 (2006).
Kastner, A., Hirsch, E. C., Herrero, M. T., Javoy-Agid, F. & Agid, Y. Immunocytochemical quantification of tyrosine hydroxylase at a cellular level in the mesencephalon of control subjects and patients with Parkinson's and Alzheimer's disease. J. Neurochem. 61, 1024–1034 (1993).
Miller, G. W. et al. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson's disease. Exp. Neurol. 156, 138–148 (1999).
Muthane, U., Yasha, T. C. & Shankar, S. K. Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India. Ann. Neurol. 43, 283–287 (1998).
Haycock, J. W. et al. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J. Neurochem. 87, 574–585 (2003).
Kish, S. J., Shannak, K. & Hornykiewicz, O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 318, 876–880 (1988).
Joyce, J. N. The Basal Ganglia Dopaminergic Systems in Normal Aging and Parkinson's Disease in Functional Neurobiology of Aging (eds Hof. P. R. & Mobbs, C. V.) 689–709 (Academic Press, San Diego,2001).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Zeng, B. Y., Medhurst, A. D., Jackson, M., Rose, S. & Jenner, P. Proteasomal activity in brain differs between species and brain regions and changes with age. Mech. Ageing Dev. 126, 760–766 (2005).
Goldman, J. E., Yen, S. H., Chiu, F. C. & Peress, N. S. Lewy bodies of Parkinson's disease contain neurofilament antigens. Science 221, 1082–1084 (1983).
McNaught, K. S., Belizaire, R., Isacson, O., Jenner, P. & Olanow, C. W. Altered proteasomal function in sporadic Parkinson's disease. Exp. Neurol. 179, 38–46 (2003).
McNaught, K. S., Belizaire, R., Jenner, P., Olanow, C. W., Isacson, O. Selective loss of 20S proteasome α-subunits in the substantia nigra pars compacta in Parkinson's disease. Neurosci. Lett. 326, 155–158 (2002).
Zhu, J. H., Kulich, S. M., Oury, T. D. & Chu, C. T. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am. J. Pathol. 161, 2087–2098 (2002).
Zhu, J. H., Guo, F., Shelburne, J., Watkins, S. & Chu, C. T. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 13, 473–481 (2003).
Corral-Debrinski, M. et al. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nature Genet. 2, 324–329 (1992).
Soong, N. W., Hinton, D. R., Cortopassi, G. & Arnheim, N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nature Genet. 2, 318–323 (1992).
Dawson, T. M. & Dawson, V. L. Molecular pathways of neurodegeneration in Parkinson's disease. Science 302, 819–822 (2003).
Gu, M. et al. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J. Neurol. Sci. 158, 24–29 (1998).
Mizuno, Y. et al. Mitochondrial energy crisis in Parkinson's disease. Adv. Neurol. 60, 282–287 (1993).
Squier, T. C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 36, 1539–1550 (2001).
Alam, Z. I. et al. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J. Neurochem. 69, 1326–1329 (1997).
Alam, Z. I. et al. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 69, 1196–1203 (1997).
Dexter, D. T. et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J. Neurochem. 52, 381–389 (1989).
Beach, T. G., Walker, R. & McGeer, E. G. Patterns of gliosis in Alzheimer's disease and aging cerebrum. Glia 2, 420–436 (1989).
Damier, P., Hirsch, E. C., Zhang, P., Agid, Y. & Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience 52, 1–6 (1993).
Forno, L. S., DeLanney, L. E., Irwin, I., Di, M. D. & Langston, J. W. Astrocytes and Parkinson's disease. Prog. Brain Res. 94, 429–436 (1992).
Sheffield, L. G. & Berman, N. E. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol. Aging 19, 47–55 (1998).
McGeer, P. L., Itagaki, S. & McGeer, E. G. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 76, 550–557 (1988).
Acknowledgements
We are grateful for the dedication and effort of all members of our investigative teams and the generous support provided by the US National Institutes of Health (NIH) awards AG10851 and NS58830 (to the Udall Center of Excellence in Parkinson's Disease Research at Michigan State University, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.H.K. is a founding scientist with a financial interest in Ceregene Inc., USA.
Related links
Glossary
- Lipofuscin
-
Autofluorescent lipid-containing residues of lysosomal digestion that accumulate in many tissues of the body with advancing age and have been termed 'age pigment'.
- Neuromelanin
-
A modified form of melanin pigment found in dopamine neurons of the substantia nigra.
- Probenicid
-
An adjuvant that, when co-administered with MPTP, blocks rapid clearance of the toxin and its metabolites, producing a progressive rodent model of parkinsonism.
- Synucleinopathy
-
An abnormal structure or quantity of α-synuclein that disrupts the function of cells.
Rights and permissions
About this article
Cite this article
Collier, T., Kanaan, N. & Kordower, J. Ageing as a primary risk factor for Parkinson's disease: evidence from studies of non-human primates. Nat Rev Neurosci 12, 359–366 (2011). https://doi.org/10.1038/nrn3039
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3039
This article is cited by
-
Dopaminergic neuron loss in mice due to increased levels of wild-type human α-Synuclein only takes place under conditions of accelerated aging
Scientific Reports (2024)
-
Gut-Brain Axis Deregulation and Its Possible Contribution to Neurodegenerative Disorders
Neurotoxicity Research (2024)
-
The Synergistic Effect Study of Lipopolysaccharide (LPS) and A53T-α-Synuclein: Intranasal LPS Exposure on the A53T-α-Synuclein Transgenic Mouse Model of Parkinson’s Disease
Molecular Neurobiology (2024)
-
Recent updates on structural insights of MAO-B inhibitors: a review on target-based approach
Molecular Diversity (2023)
-
Long-acting injectable in situ gel of rasagiline: a patented product development
Drug Delivery and Translational Research (2023)