Key Points
-
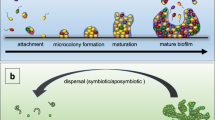
Bacterial cells growing in biofilms are physiologically distinct from free-swimming planktonic cells. For example, differences have been shown in motility, polysaccharide production, antibiotic tolerance and global proteomic and transcriptomic profiles.
-
Bacterial cells within biofilms can also be physiologically distinct from adjacent cells on a micrometre scale.
-
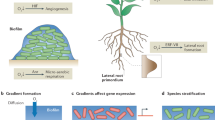
Chemical heterogeneities are established in biofilms primarily owing to bacterial metabolic activity and solute diffusion. Chemical gradients of oxygen, nutrients, bacterial waste products and bacterial signalling compounds can therefore be established, thereby generating unique environmental conditions for the cells.
-
Bacterial adaptation to chemical gradients in biofilms includes differences in gene expression and protein production. Also relevant are mutation and selection for the fittest organisms in a particular microenvironment, as well as the response that is due to stochastic gene expression.
-
Techniques for characterizing gene expression and physiological activities have been applied to bacterial biofilms to characterize the local activity of cells within biofilms. Examples that used these techniques are discussed, including the advantages and disadvantages of using particular techniques to characterize subsets of cells from within biofilms.
Abstract
Biofilms contain bacterial cells that are in a wide range of physiological states. Within a biofilm population, cells with diverse genotypes and phenotypes that express distinct metabolic pathways, stress responses and other specific biological activities are juxtaposed. The mechanisms that contribute to this genetic and physiological heterogeneity include microscale chemical gradients, adaptation to local environmental conditions, stochastic gene expression and the genotypic variation that occurs through mutation and selection. Here, we discuss the processes that generate chemical gradients in biofilms, the genetic and physiological responses of the bacteria as they adapt to these gradients and the techniques that can be used to visualize and measure the microscale physiological heterogeneities of bacteria in biofilms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev. Microbiol. 2, 95–108 (2004).
Drenkard, E. & Ausubel, F. M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416, 740–743 (2002).
Mah, T. F. et al. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426, 306–310 (2003).
Bjarnsholt, T. et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151, 373–383 (2005).
Jesaitis, A. J. et al. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171, 4329–4339 (2003).
Bryers, J. D. in Biofilms (eds Characklis, W. G. & Marshall, K. C.) 733–773 (Wiley-Intersciences, New York, 1990).
Bryers, J. D. & Characklis, W. G. in Biofilms (eds Characklis, W. G. & Marshall, K. C.) 671–696 (Wiley-Intersciences, New York, 1990).
Belas, R., Simon, M. & Silverman, M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167, 210–218 (1986).
McCarter, L. & Silverman, M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol. Microbiol. 4, 1057–1062 (1990).
Davies, D. G. & Geesey, G. G. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61, 860–867 (1995).
Dalton, H. M. et al. Substratum-induced morphological changes in a marine bacterium and their relevance to biofilm structure. J. Bacteriol. 176, 6900–6906 (1994).
An, D. & Parsek, M. R. The promise and peril of transcriptional profiling in biofilm communities. Curr. Opin. Microbiol. 10, 292–296 (2007).
Sauer, K., Camper, A. K., Ehrlich, G. D., Costerton, J. W. & Davies, D. G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184, 1140–1154 (2002). Demonstrated that P. aeruginosa undergoes a range of physiological changes during the initiation, development, maturation and dispersal of biofilms, and that each physiological state is accompanied by dramatic changes in the proteome profiles of the cells.
Southey-Pillig, C. J., Davies, D. G. & Sauer, K. Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J. Bacteriol. 187, 8114–8126 (2005).
O'Toole, G. A. & Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28, 449–461 (1998). Describes a microtitre dish screening assay that was used to identify transposon mutants that are unable to form biofilms, thereby linking distinct genetic loci to biofilm formation.
Patrauchan, M. A., Sarkisova, S. & Franklin, M. J. Strain specific proteome responses of Pseudomonas aeruginosa to biofilm-associated growth and to calcium. Microbiology 153, 3838–3851 (2007).
Glud, R. N., Ramsing, N. B. & Revsbech, N. P. Photosynthesis and photosynthesis-coupled respiration in natural biofilms quantified with oxygen microsensors. J. Phycol. 28, 51–60 (1992).
Ramsing, N. B., Kuhl, M. & Jorgensen, B. B. Distribution of sulfate-reducing bacteria, O2, and H2S in photosynthetic biofilms determined by oligonucleotide probes and microelectrodes. Appl. Environ. Microbiol. 59, 3840–3849 (1993). Reports microelectrode measurements of concentration gradients in biofilms, and integrates these with ecological analysis by the use of FISH probes.
De Beer, D., Stoodley, P., Roe, F. & Lewandowski, Z. Effects of biofilm structure on oxygen distribution and mass transport. Biotechnol. Bioeng. 43, 1131–1138 (2004).
Zhang, T., Fu, Y. & Bishop, P. Competition for substrate and space in biofilms. Water Environ. Res. 67, 992–1003 (1995).
Schramm, A. et al. Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes. Appl. Environ. Microbiol. 62, 4641–4647 (1996).
Xu, K. D., Stewart, P. S., Xia, F., Huang, C. T. & McFeters, G. A. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 64, 4035–4039 (1998).
Okabe, S., Satoh, H. & Watanabe, Y. In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl. Environ. Microbiol. 65, 3182–3191 (1999).
Rani, S. A. et al. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J. Bacteriol. 189, 4223–4233 (2007). Experimental demonstration of spatial heterogeneity in oxygen concentration and anabolic protein and DNA synthesis.
Allewalt, J. P., Bateson, M. M., Revsbech, N. P., Slack, K. & Ward, D. M. Effect of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the octopus spring microbial mat community of Yellowstone National Park. Appl. Environ. Microbiol. 72, 544–550 (2006).
Ward, D. M. Microbial diversity in natural environments: focusing on fundamental questions. Antonie Van Leeuwenhoek 90, 309–324 (2006).
Kolenbrander, P. E. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54, 413–437 (2000).
Stewart, P. S. Diffusion in biofilms. J. Bacteriol. 185, 1485–1491 (2003).
Damgaard, L. R., Nielsen, L. P. & Revsbech, N. P. Methane microprofiles in a sewage biofilm determined with a microscale biosensor. Water Res. 35, 1379–1386 (2001).
De Beer, D., Schramm, A., Santegoeds, C. M. & Kuhl, M. A nitrite microsensor for profiling environmental biofilms. Appl. Environ. Microbiol. 63, 973–977 (1997).
Kuhl, M., Steuckart, C., Eickert, G. & Jeroschewski, P. A H2S microsensor for profiling biofilms and sediments: application in an acidic lake sediment. Aquat. Microb. Ecol. 15, 201–209 (1998).
Okabe, S., Itoh, T., Satoh, H. & Watanabe, Y. Analyses of spatial distributions of sulfate-reducing bacteria and their activity in aerobic wastewater biofilms. Appl. Environ. Microbiol. 65, 5107–5116 (1999).
De Beer, D., Glud, A., Epping, E. & Kuhl, M. A fast-responding CO2 microelectrode for profiling sediments, microbial mats, and biofilms. Limnol. Oceanogr. 42, 1590–1600 (1997).
Beyenal, H., Davis, C. & Lewandowski, Z. An improved Severinghaus-type carbon dioxide microelectrode for use in biofilms. Sens Actuators B 97, 202–210 (2004).
Liu, X., Roe, F., Jesaitis, A. J. & Lewandowski, Z. Resistance of biofilms to the catalase inhibitor 3-amino-1,2,4-triazole. Biotechnol. Bioeng. 59, 156–162 (1998).
De Beer, D., Srinivasan, R. & Stewart, P. S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 60, 4339–4344 (1994).
Jang, A., Szabo, J., Hosni, A. A., Coughlin, M. & Bishop, P. L. Measurement of chlorine dioxide penetration in dairy process pipe biofilms during disinfection. Appl. Microbiol. Biotechnol. 72, 368–376 (2006).
De Beer, D., Huisman, J., Van den Heuvel, J. & Ottengraf, S. The effect of pH profiles in methanogenic aggregates on the kinetics of acetate conversion. Water Res. 26, 1329–1336 (1992).
Huang, C. T., Yu, F. P., McFeters, G. & Stewart, P. S. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl. Environ. Microbiol. 61, 2252–2256 (1995).
Wentland, E., Stewart, P. S., Huang, C. T. & McFeters, G. A. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol. Prog. 12, 316–321 (1996).
Huang, C. T., Xu, K. D., McFeters, G. A. & Stewart, P. S. Spatial patterns of alkaline phosphatase expression within bacterial colonies and biofilms in response to phosphate starvation. Appl. Environ. Microbiol. 64, 1526–1531 (1998).
Kuhl, M. & Jorgensen, B. B. Microsensor measurements of sulfate reduction and sulfide oxidation in compact microbial communities of aerobic biofilms. Appl. Environ. Microbiol. 58, 1164–1174 (1992).
Otto, K. & Silhavy, T. J. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl Acad. Sci. USA 99, 2287–2292 (2002).
Kolenbrander, P. E. et al. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 66, 486–505 (2002).
Stover, C. K. et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406, 959–964 (2000).
Allegrucci, M. & Sauer, K. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 189, 2030–2038 (2007).
Hansen, S. K., Rainey, P. B., Haagensen, J. A. & Molin, S. Evolution of species interactions in a biofilm community. Nature 445, 533–536 (2007).
Valle, J., Vergara-Irigaray, M., Merino, N., Penades, J. R. & Lasa, I. σB regulates IS256-mediated Staphylococcus aureus biofilm phenotypic variation. J. Bacteriol. 189, 2886–2896 (2007).
Yildiz, F. H. & Schoolnik, G. K. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl Acad. Sci. USA 96, 4028–4033 (1999).
McEllistrem, M. C., Ransford, J. V. & Khan, S. A. Characterization of in vitro biofilm-associated pneumococcal phase variants of a clinically relevant serotype 3 clone. J. Clin. Microbiol. 45, 97–101 (2007).
Koh, K. S. et al. Phenotypic diversification and adaptation of Serratia marcescens MG1 biofilm-derived morphotypes. J. Bacteriol. 189, 119–130 (2007).
Kirisits, M. J., Prost, L., Starkey, M. & Parsek, M. R. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 71, 4809–4821 (2005).
Haussler, S. Biofilm formation by the small colony variant phenotype of Pseudomonas aeruginosa. Environ. Microbiol. 6, 546–551 (2004).
Nguyen, D. & Singh, P. K. Evolving stealth: genetic adaptation of Pseudomonas aeruginosa during cystic fibrosis infections. Proc. Natl Acad. Sci. USA 103, 8305–8306 (2006).
Hansen, S. K. et al. Characterization of a Pseudomonas putida rough variant evolved in a mixed-species biofilm with Acinetobacter sp. strain C6. J. Bacteriol. 189, 4932–4943 (2007).
Boles, B. R., Thoendel, M. & Singh, P. K. Self-generated diversity produces “insurance effects” in biofilm communities. Proc. Natl Acad. Sci. USA 101, 16630–16635 (2004). Demonstrated that P. aeruginosa undergoes genetic diversification during short-term biofilm growth, which might allow increased resistance of the population to environmental stresses.
Cooper, T. F., Beaumont, H. J. & Rainey, P. B. Biofilm diversity as a test of the insurance hypothesis. Microbiology 151, 2815–2816; discussion 2816–2818 (2005).
Deziel, E., Comeau, Y. & Villemur, R. Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J. Bacteriol. 183, 1195–1204 (2001).
Baty, A. M. et al. Differentiation of chitinase-active and non-chitinase-active subpopulations of a marine bacterium during chitin degradation. Appl. Environ. Microbiol. 66, 3566–3573 (2000). Used a gfp reporter to the chitinase promoter to demonstrate that biofilms of the marine bacterium Pseudoalteromonas sp. resulted in two distinct subpopulations with respect to chitinase activity; this seems to have been due to stochastic gene expression.
Baty, A. M., Eastburn, C. C., Techkarnjanaruk, S., Goodman, A. E. & Geesey, G. G. Spatial and temporal variations in chitinolytic gene expression and bacterial biomass production during chitin degradation. Appl. Environ. Microbiol. 66, 3574–3585 (2000).
Hasty, J., Pradines, J., Dolnik, M. & Collins, J. J. Noise-based switches and amplifiers for gene expression. Proc. Natl Acad. Sci. USA 97, 2075–2080 (2000).
McAdams, H. H. & Arkin, A. Stochastic mechanisms in gene expression. Proc. Natl Acad. Sci. USA 94, 814–819 (1997).
Elowitz, M. B., Levine, A. J., Siggia, E. D. & Swain, P. S. Stochastic gene expression in a single cell. Science 297, 1183–1186 (2002).
Chai, Y., Chu, F., Kolter, R. & Losick, R. Bistability and biofilm formation in Bacillus subtilis. Mol. Microbiol. 67, 254–263 (2007). Demonstrated that matrix polymer production in B. subtilis is under the control of a bistable mechanism that involves the repressor SinR and its anti-repressor SinI.
Branda, S. S., Chu, F., Kearns, D. B., Losick, R. & Kolter, R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59, 1229–1238 (2006).
Branda, S. S., Gonzalez-Pastor, J. E., Ben-Yehuda, S., Losick, R. & Kolter, R. Fruiting body formation by Bacillus subtilis. Proc. Natl Acad. Sci. USA 98, 11621–11626 (2001). Showed that, when cultured in biofilms, B. subtilis spore formation is spatially organized and occurs in structures that resemble fruiting bodies.
Keren, I., Kaldalu, N., Spoering, A., Wang, Y. & Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230, 13–18 (2004).
Lewis, K. Persister cells, dormancy and infectious disease. Nature Rev. Microbiol. 5, 48–56 (2007).
Levin, B. R. & Rozen, D. E. Non-inherited antibiotic resistance. Nature Rev. Microbiol. 4, 556–562 (2006).
Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L. & Leibler, S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (2004). A microfluidics approach was used to investigate the pre-existing heterogeneity in growth rates of bacteria at the single-cell level. Cells that had reduced growth rates were linked with persistence to antibiotic challenge.
Kussell, E., Kishony, R., Balaban, N. Q. & Leibler, S. Bacterial persistence: a model of survival in changing environments. Genetics 169, 1807–1814 (2005).
Correia, F. F. et al. Kinase activity of overexpressed HipA is required for growth arrest and multidrug tolerance in Escherichia coli. J. Bacteriol. 188, 8360–8367 (2006).
Korch, S. B. & Hill, T. M. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation. J. Bacteriol. 188, 3826–3836 (2006).
Li, Y. & Zhang, Y. PhoU is a persistence switch involved in persister formation and tolerance to multiple antibiotics and stresses in Escherichia coli. Antimicrob. Agents Chemother. 51, 2092–2099 (2007).
Spoering, A. L., Vulic, M. & Lewis, K. GlpD and PlsB participate in persister cell formation in Escherichia coli. J. Bacteriol. 188, 5136–5144 (2006).
Rodriguez, G. G., Phipps, D., Ishiguro, K. & Ridgway, H. F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Microbiol. 58, 1801–1808 (1992).
Schaule, G., Flemming, H. C. & Ridgway, H. F. Use of 5-cyano-2,3-ditolyl tetrazolium chloride for quantifying planktonic and sessile respiring bacteria in drinking water. Appl. Environ. Microbiol. 59, 3850–3857 (1993).
Zheng, Z. & Stewart, P. S. Growth limitation of Staphylococcus epidermidis in biofilms contributes to rifampin tolerance. Biofilms 1, 31–45 (2004).
Nielsen, J. L., Aquino de Muro, M. & Nielsen, P. H. Evaluation of the redox dye 5-cyano-2,3-tolyl-tetrazolium chloride for activity studies by simultaneous use of microautoradiography and fluorescence in situ hybridization. Appl. Environ. Microbiol. 69, 641–643 (2003).
Ullrich, S., Karrasch, B., Hoppe, H., Jeskulke, K. & Mehrens, M. Toxic effects on bacterial metabolism of the redox dye 5-cyano-2,3-ditolyl tetrazolium chloride. Appl. Environ. Microbiol. 62, 4587–4593 (1996).
Smith, J. J. & McFeters, G. A. Effects of substrates and phosphate on INT (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride) and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) reduction in Escherichia coli. J. Appl. Bacteriol. 80, 209–215 (1996).
Korber, D. R., Choi, A., Wolfaardt, G. M., Ingham, S. C. & Caldwell, D. E. Substratum topography influences susceptibility of Salmonella enteritidis biofilms to trisodium phosphate. Appl. Environ. Microbiol. 63, 3352–3358 (1997).
Lindsay, D., Brozel, V. S., Mostert, J. F. & von Holy, A. Differential efficacy of a chlorine dioxide-containing sanitizer against single species and binary biofilms of a dairy-associated Bacillus cereus and a Pseudomonas fluorescens isolate. J. Appl. Microbiol. 92, 352–361 (2002).
Hentzer, M. et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22, 3803–3815 (2003).
Teitzel, G. M. & Parsek, M. R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69, 2313–2320 (2003).
Hope, C. K. & Wilson, M. Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob. Agents Chemother. 48, 1461–1468 (2004).
Barraud, N. et al. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 188, 7344–7353 (2006).
Berney, M., Hammes, F., Bosshard, F., Weilenmann, H. U. & Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl. Environ. Microbiol. 73, 3283–3290 (2007).
Biggerstaff, J. P. et al. New methodology for viability testing in environmental samples. Mol. Cell. Probes 20, 141–146 (2006).
Stocks, S. M. Mechanism and use of the commercially available viability stain, BacLight. Cytometry A 61, 189–195 (2004).
Suci, P. A. & Tyler, B. J. Action of chlorhexidine digluconate against yeast and filamentous forms in an early-stage Candida albicans biofilm. Antimicrob. Agents Chemother. 46, 3522–3531 (2002).
Banin, E., Brady, K. M. & Greenberg, E. P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 72, 2064–2069 (2006).
Haagensen, J. A. et al. Differentiation and distribution of colistin- and sodium dodecyl sulfate-tolerant cells in Pseudomonas aeruginosa biofilms. J. Bacteriol. 189, 28–37 (2007).
Kaneko, Y., Thoendel, M., Olakanmi, O., Britigan, B. E. & Singh, P. K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 117, 877–888 (2007). Together with reference 93, demonstrated the differing antimicrobial susceptibility of spatially segregated populations within a biofilm.
Auschill, T. M. et al. Spatial distribution of vital and dead microorganisms in dental biofilms. Arch. Oral Biol. 46, 471–476 (2001).
Honraet, K., Goetghebeur, E. & Nelis, H. J. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J. Microbiol. Methods 63, 287–295 (2005).
Hamasaki, K., Taniguchi, A., Tada, Y., Long, R. A. & Azam, F. Actively growing bacteria in the inland sea of Japan, identified by combined bromodeoxyuridine immunocapture and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 73, 2787–2798 (2007).
Hjort, K., Lembke, A., Speksnijder, A., Smalla, K. & Jansson, J. K. Community structure of actively growing bacterial populations in plant pathogen suppressive soil. Microb. Ecol. 53, 399–413 (2007).
Jackson, K. D., Starkey, M., Kremer, S., Parsek, M. R. & Wozniak, D. J. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186, 4466–4475 (2004).
Friedman, L. & Kolter, R. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186, 4457–4465 (2004).
Nivens, D. E., Ohman, D. E., Williams, J. & Franklin, M. J. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 183, 1047–1057 (2001).
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C. & Mattick, J. S. Extracellular DNA required for bacterial biofilm formation. Science 295, 1487 (2002).
Allesen-Holm, M. et al. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59, 1114–1128 (2006).
Sarkisova, S., Patrauchan, M. A., Berglund, D., Nivens, D. E. & Franklin, M. J. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 187, 4327–4337 (2005).
Neu, T., Swerhone, G. D. & Lawrence, J. R. Assessment of lectin-binding analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 147, 299–313 (2001).
Lawrence, J. R., Swerhone, G. D., Kuhlicke, U. & Neu, T. R. In situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol. 53, 450–458 (2007).
Laue, H. et al. Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiology 152, 2909–2918 (2006).
Strathmann, M., Wingender, J. & Flemming, H. C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods 50, 237–248 (2002).
Sternberg, C. et al. Distribution of bacterial growth activity in flow-chamber biofilms. Appl. Environ. Microbiol. 65, 4108–4117 (1999).
Werner, E. et al. Stratified growth in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 70, 6188–6196 (2004).
Teal, T. K., Lies, D. P., Wold, B. J. & Newman, D. K. Spatiometabolic stratification of Shewanella oneidensis biofilms. Appl. Environ. Microbiol. 72, 7324–7330 (2006). Used the unstable gfp reporter gene in S. oneidensis to demonstrate the reproducible stratification of metabolic programming to local environmental conditions and biofilm developmental processes.
Walters, M. C., Roe, F., Bugnicourt, A., Franklin, M. J. & Stewart, P. S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47, 317–323 (2003).
Borriello, G., Richards, L., Ehrlich, G. D. & Stewart, P. S. Arginine or nitrate enhances antibiotic susceptibility of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 50, 382–384 (2006).
Bagge, N. et al. Dynamics and spatial distribution of β-lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 48, 1168–1174 (2004).
De Kievit, T. R. & Iglewski, B. H. Quorum sensing, gene expression, and Pseudomonas biofilms. Methods Enzymol. 310, 117–128 (1999).
Lequette, Y. & Greenberg, E. P. Timing and localization of rhamnolipid synthesis gene expression in Pseudomonas aeruginosa biofilms. J. Bacteriol. 187, 37–44 (2005).
Yang, L. et al. Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 153, 1318–1328 (2007).
Yarwood, J. M., Bartels, D. J., Volper, E. M. & Greenberg, E. P. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 186, 1838–1850 (2004). Used a reporter gene in Staphylococcus aureus to follow complex spatial and temporal patterns of expression of a quorum-sensing gene.
De Kievit, T. R. et al. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 45, 1761–1770 (2001). Used reporter genes in P. aeruginosa to quantify and map spatial and temporal patterns of multidrug efflux-pump expression in biofilms.
Uppuluri, P., Sarmah, B. & Chaffin, W. L. Candida albicans SNO1 and SNZ1 expressed in stationary-phase planktonic yeast cells and base of biofilm. Microbiology 152, 2031–2038 (2006).
Moller, S. et al. In situ gene expression in mixed-culture biofilms: evidence of metabolic interactions between community members. Appl. Environ. Microbiol. 64, 721–732 (1998).
Klausen, M., Aaes-Jorgensen, A., Molin, S. & Tolker-Nielsen, T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50, 61–68 (2003). Used fluorescent-protein-labelled wild-type and motility-mutant strains of P. aeruginosa to show the role of cell migration and type IV pili in biofilm developmental processes.
Amann, R. I. Fluorescently labelled, rRNA-targeted oligonucleotide probes in the study of microbial ecology. Mol. Ecol. 4, 543–554 (1995).
DeLong, E. F., Wickham, G. S. & Pace, N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science 243, 1360–1363 (1989).
Moller, S., Pedersen, A. R., Poulsen, L. K., Arvin, E. & Molin, S. Activity and three-dimensional distribution of toluene-degrading Pseudomonas putida in a multispecies biofilm assessed by quantitative in situ hybridization and scanning confocal laser microscopy. Appl. Environ. Microbiol. 62, 4632–4640 (1996).
Xu, K. D., McFeters, G. A. & Stewart, P. S. Biofilm resistance to antimicrobial agents. Microbiology 146, 547–549 (2000).
Jang, A., Okabe, S., Watanabe, Y., Kim, I. & Bishop, P. Measurement of growth rate of ammonia oxidizing bacteria in partially submerged rotating biological contactor by fluorescent in situ hybridization (FISH). J. Environ. Sci. Eng. 4, 413–420 (2005).
Lehtola, M. J., Torvinen, E., Miettinen, I. T. & Keevil, C. W. Fluorescence in situ hybridization using peptide nucleic acid probes for rapid detection of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. paratuberculosis in potable-water biofilms. Appl. Environ. Microbiol. 72, 848–853 (2006).
Ferrari, B. C., Tujula, N., Stoner, K. & Kjelleberg, S. Catalyzed reporter deposition-fluorescence in situ hybridization allows for enrichment-independent detection of microcolony-forming soil bacteria. Appl. Environ. Microbiol. 72, 918–922 (2006).
Pernthaler, A., Pernthaler, J. & Amann, R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68, 3094–3101 (2002).
Fazi, S., Amalfitano, S., Pernthaler, J. & Puddu, A. Bacterial communities associated with benthic organic matter in headwater stream microhabitats. Environ. Microbiol. 7, 1633–1640 (2005).
Magnuson, T. S., Neal, A. L. & Geesey, G. G. Combining in situ reverse transcriptase polymerase chain reaction, optical microscopy, and X-ray photoelectron spectroscopy to investigate mineral surface-associated microbial activities. Microb. Ecol. 48, 578–588 (2004).
Wagner, M., Nielsen, P. H., Loy, A., Nielsen, J. L. & Daims, H. Linking microbial community structure with function: fluorescence in situ hybridization-microautoradiography and isotope arrays. Curr. Opin. Biotechnol. 17, 83–91 (2006).
Huang, W. E. et al. Raman-FISH: combining stable-isotope Raman spectroscopy and fluorescence in situ hybridization for the single cell analysis of identity and function. Environ. Microbiol. 9, 1878–1889 (2007).
Lee, N. et al. Combination of fluorescent in situ hybridization and microautoradiography — a new tool for structure-function analyses in microbial ecology. Appl. Environ. Microbiol. 65, 1289–1297 (1999).
Ouverney, C. C. & Fuhrman, J. A. Combined microautoradiography-16S rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl. Environ. Microbiol. 65, 1746–1752 (1999).
Kindaichi, T., Ito, T. & Okabe, S. Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl. Environ. Microbiol. 70, 1641–1650 (2004).
Ito, T., Nielsen, J. L., Okabe, S., Watanabe, Y. & Nielsen, P. H. Phylogenetic identification and substrate uptake patterns of sulfate-reducing bacteria inhabiting an oxic–anoxic sewer biofilm determined by combining microautoradiography and fluorescent in situ hybridization. Appl. Environ. Microbiol. 68, 356–364 (2002).
Bonner, R. F. et al. Laser capture microdissection: molecular analysis of tissue. Science 278, 1481–1483 (1997).
Emmert-Buck, M. R. et al. Laser capture microdissection. Science 274, 998–1001 (1996).
Lenz, A. P., Williamson, K., Pitts, B., Stewart, P. S. & Franklin, M. J. in Abstr. Gen. Meet. Am. Soc. Microbiol. (American Society of Microbiology, Washington D.C., 2007).
Rani, S. A., Pitts, B. & Stewart, P. S. Rapid diffusion of fluorescent tracers into Staphylococcus epidermidis biofilms visualized by time lapse microscopy. Antimicrob. Agents Chemother. 49, 728–732 (2005).
Stewart, P. S. et al. Observations of cell cluster hollowing in Staphylococcus epidermidis biofilms. Lett. Appl. Microbiol. 44, 454–457 (2007).
Seymour, J. D., Codd, S. L., Gjersing, E. L. & Stewart, P. S. Magnetic resonance microscopy of biofilm structure and impact on transport in a capillary bioreactor. J. Magn. Reson. 167, 322–327 (2004).
Acknowledgements
Work in the laboratory of P.S.S. has been supported by National Institutes of Health grant 5R01GM67245 and an award from the W. M. Keck Foundation. M.J.F.'s work on this topic is supported by Public Health Service grant AI-065906 from the National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Montana State University's Center for Biofilm Engineering Resource Library (biofilm movies)
Glossary
- Extracellular polymeric substance
-
A polymer, such as a polysaccharide, protein or nucleic acid, that is secreted by bacteria and forms a hydrated gel-like slime. Extracellular polymeric substances hold the biofilm together, and might serve other functions, such as nutrient trapping and protection from antimicrobial challenges.
- Reaction–diffusion theory.
-
A mathematical analysis of the distribution of a chemical solute in space and time that results from the interaction of two processes: reaction of the solute and its transport by diffusion. Reaction–diffusion interactions generate spatial gradients in the concentration of reacting solutes.
- Persister cell
-
A metabolically quiescent cell that neither grows nor dies when exposed to cidal concentrations of antimicrobial compounds.
- Peptide nucleic acid probe–FISH
-
Sequence-specific identification of nucleic acids using fluorescently labelled probes that contain peptide backbones. Useful for identifying individual cells in biofilms, generally by probing specific ribosomal RNA sequences.
- Catalyzed reporter deposition–FISH
-
A method for increasing the sensitivity of FISH by using horseradish peroxidase-labelled oligonucleotide probes. The enzyme catalyzes the deposition of tyramine molecules, which results in fluorescent signal amplification at the site of probe hybridization.
- In situ reverse-transcription polymerase chain reaction
-
A method to convert mRNA to cDNA and amplify the cDNA from cells that are fixed on microscope slides. Gene expression from individual cells is then assayed by using sequence-specific fluorescent probes that hybridize to the amplified product.
- Microautoradiography
-
A method in which the activity of individual cells is determined by assaying incorporation of a radiolabelled substrate into cell material. Cell activity is determined by exposing labelled cells to photographic emulsion and quantifying exposed silver grains adjacent to the cells.
- Laser capture microdissection microscopy
-
A microscopic method in which a laser is used to excise subsets of cells from the surrounding population. The excised cells can then be isolated for further analysis by laser catapulting.
Rights and permissions
About this article
Cite this article
Stewart, P., Franklin, M. Physiological heterogeneity in biofilms. Nat Rev Microbiol 6, 199–210 (2008). https://doi.org/10.1038/nrmicro1838
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1838
This article is cited by
-
Microfluidics for adaptation of microorganisms to stress: design and application
Applied Microbiology and Biotechnology (2024)
-
“Sharing the matrix” – a cooperative strategy for survival in Salmonella enterica serovar Typhimurium
BMC Microbiology (2023)
-
Effects of DJK-5 and chlorhexidine on exopolysaccharide volume and pH in oral biofilms
BMC Oral Health (2023)
-
Antimicrobial efficacy of self-locomotive manganese oxide nanozyme-doped diatom microbubbler on orthodontic brackets in vitro
BMC Oral Health (2023)
-
Long-term label-free assessments of individual bacteria using three-dimensional quantitative phase imaging and hydrogel-based immobilization
Scientific Reports (2023)