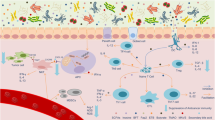
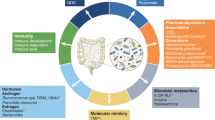
Key Points
-
The evolution of cancer has been linked to shifts in the microbiome.
-
It will be indispensable to identify individual strains and clones (rather than phyla and genera) that have optimal anticancer effects. For this, culturomics will be superior to deep-sequencing approaches.
-
Therapeutic manipulation of the cancer-associated microbiome may be obtained by faecal microbiota transplantation, antibiotic treatment, prebiotics that favour the expansion of useful bacteria, dietary interventions or drugs that alter the composition of the gut flora.
-
In preclinical models, defined strains of live microbial agents may be used to stimulate immunosurveillance against cancers, either alone or in combination with cancer therapeutics.
-
Bacterial products that have potential antineoplastic or immunostimulatory properties include bacterial toxins, microbial ligands of pattern recognition receptors, as well as bacterial metabolites, including butyrate, polyamines and pyridoxine.
-
Drugs that modify bacterial metabolism are being developed with the scope of inhibiting the production of carcinogenic products.
Abstract
The human gut microbiome modulates many host processes, including metabolism, inflammation, and immune and cellular responses. It is becoming increasingly apparent that the microbiome can also influence the development of cancer. In preclinical models, the host response to cancer treatment has been improved by modulating the gut microbiome; this is known to have an altered composition in many diseases, including cancer. In addition, cancer treatment with microbial agents or their products has the potential to shrink tumours. However, the microbiome could also negatively influence cancer prognosis through the production of potentially oncogenic toxins and metabolites by bacteria. Thus, future antineoplastic treatments could combine the modulation of the microbiome and its products with immunotherapeutics and more conventional approaches that directly target malignant cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pevsner-Fischer, M. et al. Role of the microbiome in non-gastrointestinal cancers. World J. Clin. Oncol. 7, 200–213 (2016).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Honda, K. & Littman, D. R. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016).
Gilbert, J. A. et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 535, 94–103 (2016).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004). A key paper on the definition of immunosurveillance in oncoimmunology.
Nakatsu, G. et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 6, 8727 (2015).
Mitra, A. et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 5, 16865 (2015).
Yu, G. et al. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 17, 163 (2016).
Kilkkinen, A. et al. Antibiotic use predicts an increased risk of cancer. Int. J. Cancer 123, 2152–2155 (2008). This paper provides the first clinical evidence that links modifications of the microbiota to cancer.
Zitvogel, L., Ayyoub, M., Routy, B. & Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016).
Guerrero-Preston, R. et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 7, 51320–51334 (2016).
Piyathilake, C. J. et al. Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev. Res. (Phila.) 9, 357–366 (2016).
Jacquelot, N. et al. Chemokine receptor patterns in lymphocytes mirror metastatic spreading in melanoma. J. Clin. Invest. 126, 921–937 (2016).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013). This paper highlights the importance of an intact microbiota for CTX activity, and the pivotal role of E. hirae translocation following CTX treatment to induce pathogenic T H 17 cells.
Tsilimigras, M. C. B. & Fodor, A. A. Compositional data analysis of the microbiome: fundamentals, tools, and challenges. Ann. Epidemiol. 26, 330–335 (2016).
Hugon, P., Lagier, J.-C., Colson, P., Bittar, F. & Raoult, D. Repertoire of human gut microbes. Microb. Pathog. http://dx.doi.org/10.1016/j.micpath.2016.06.020 (2016).
Hieken, T. J. et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 6, 30751 (2016).
Rahbar, A. et al. Discordant humoral and cellular immune responses to cytomegalovirus (CMV) in glioblastoma patients whose tumors are positive for CMV. Oncoimmunology 4, e982391 (2015).
Chan, A. A. et al. Characterization of the microbiome of nipple aspirate fluid of breast cancer survivors. Sci. Rep. 6, 28061 (2016).
Drewes, J. L., Housseau, F. & Sears, C. L. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br. J. Cancer 115, 273–280 (2016).
Daillère, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). This paper details the mandatory role of the gut microbiota (specifically, B. fragilis ) in CTLA4-mediated immune anticancer activity, and the protective effect on immune-related colitis.
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016). This paper examines the link between the gut microbiota and immune checkpoint inhibitor blockade, and immune related toxicities in patients with metastatic melanoma.
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015). This study shows that Bifidobacterium spp. promote maturation of intratumoural dendritic cells that allow the expansion of anticancer T cells after programmed cell death 1 ligand 1 (PDL1) treatment in mice.
Chevalier, C. et al. Gut microbiota orchestrates energy homeostasis during cold. Cell 163, 1360–1374 (2015).
McKenney, P. T. & Pamer, E. G. From hype to hope: the gut microbiota in enteric infectious disease. Cell 163, 1326–1332 (2015).
Kamdar, K. et al. Genetic and metabolic signals during acute enteric bacterial infection alter the microbiota and drive progression to chronic inflammatory disease. Cell Host Microbe 19, 21–31 (2016).
Schmidt, C. Mental health: thinking from the gut. Nature 518, S12–S15 (2015).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109.e6 (2015).
Kakihana, K. et al. Fecal microbiota transplantation for patients with steroid-resistant/dependent acute graft-versus-host disease of the gut. Blood 128, 2083–2088 (2016). This study provides the first clinical demonstration that faecal microbiota transplantation can be safely used and improves outcomes in patients with steroid-refractory gastrointestinal graft-versus-host disease.
Bel, S. et al. Reprogrammed and transmissible intestinal microbiota confer diminished susceptibility to induced colitis in TMF−/− mice. Proc. Natl Acad. Sci. USA 111, 4964–4969 (2014).
Kwa, M., Plottel, C. S., Blaser, M. J. & Adams, S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl Cancer Inst. 108, djw029 (2016).
Dal Peraro, M. & van der Goot, F. G. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 (2016).
Pamer, E. G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 352, 535–538 (2016).
Cougnoux, A. et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63, 1932–1942 (2014).
Cougnoux, A. et al. Small-molecule inhibitors prevent the genotoxic and protumoural effects induced by colibactin-producing bacteria. Gut 65, 278–285 (2016).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
Wallace, B. D. et al. Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chem. Biol. 22, 1238–1249 (2015).
Carmody, R. N. & Turnbaugh, P. J. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J. Clin. Invest. 124, 4173–4181 (2014).
Hu, Y. et al. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 37, 366–375 (2016).
Masumoto, S. et al. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 6, 31208 (2016).
Gallus, S. et al. Does an apple a day keep the oncologist away? Ann. Oncol. 16, 1841–1844 (2005).
Taper, H. S. & Roberfroid, M. B. Nontoxic potentiation of cancer chemotherapy by dietary oligofructose or inulin. Nutr. Cancer 38, 1–5 (2000).
Schoener, C. A., Carillo-Conde, B., Hutson, H. N. & Peppas, N. A. An inulin and doxorubicin conjugate for improving cancer therapy. J. Drug Deliv. Sci. Technol. 23, 111–118 (2013).
Cabreiro, F. et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239 (2013).
Dao, M. C. et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436 (2016).
Vernieri, C. et al. Targeting cancer metabolism: dietary and pharmacologic interventions. Cancer Discov. 6, 1315–1333 (2016).
Crawford, P. A. et al. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl Acad. Sci. USA 106, 11276–11281 (2009).
Becattini, S., Taur, Y. & Pamer, E. G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22, 458–478 (2016).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016). This study illustrates the links between caloric restriction mimetics, autophagy and cancer.
Di Biase, S. et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146 (2016).
Nauts, H. C., Swift, W. E. & Coley, B. L. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 6, 205–216 (1946). This paper details the pioneering work of B. L. Coley, who 70 years ago already recognized the anticancer effects of bacteria against sarcoma.
Babjuk, M. et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur. Urol. 64, 639–653 (2013).
Böhle, A. & Brandau, S. Immune mechanisms in bacillus Calmette–Guerin immunotherapy for superficial bladder cancer. J. Urol. 170, 964–969 (2003).
Zbar, B., Bernstein, I., Tanaka, T. & Rapp, H. J. Tumor immunity produced by the intradermal inoculation of living tumor cells and living Mycobacterium bovis (strain BCG). Science 170, 1217–1218 (1970).
Aragón, F., Carino, S., Perdigón, G. & de Moreno de LeBlanc, A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J. Immunother. 38, 185–196 (2015).
Hu, J. et al. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J. Biosci. 40, 269–279 (2015).
Kato, I., Endo, K. & Yokokura, T. Effects of oral administration of Lactobacillus casei on antitumor responses induced by tumor resection in mice. Int. J. Immunopharmacol. 16, 29–36 (1994).
Cai, S. et al. Lactobacillus rhamnosus GG activation of dendritic cells and neutrophils depends on the dose and time of exposure. J. Immunol. Res. 2016, 7402760 (2016).
Khazaie, K. et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl Acad. Sci. USA 109, 10462–10467 (2012).
Konishi, H. et al. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 7, 12365 (2016).
Lenoir, M. et al. Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J. Gastroenterol. 51, 862–873 (2016).
Lee, J.-W. et al. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactobacillus casei and Bifidobacterium longum. J. Vet. Sci. 5, 41–48 (2004).
Baldwin, C. et al. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr. Cancer 62, 371–378 (2010).
Takagi, A. et al. Relationship between the in vitro response of dendritic cells to Lactobacillus and prevention of tumorigenesis in the mouse. J. Gastroenterol. 43, 661–669 (2008).
Varian, B. J. et al. Beneficial bacteria inhibit cachexia. Oncotarget 7, 11803–11816 (2016).
Li, J. et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl Acad. Sci. USA 113, E1306–E1315 (2016).
Geis, A. L. et al. Regulatory T-cell response to enterotoxigenic Bacteroides fragilis colonization triggers IL17-dependent colon carcinogenesis. Cancer Discov. 5, 1098–1109 (2015).
Wang, Q. et al. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 203, 2853–2863 (2006).
Surana, N. K. & Kasper, D. L. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol. Rev. 245, 13–26 (2012).
Din, M. O. et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85 (2016).
Moura-Alves, P. et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 512, 387–392 (2014).
Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636 (2016).
Malla, S., Niraula, N. P., Singh, B., Liou, K. & Sohng, J. K. Limitations in doxorubicin production from Streptomyces peucetius. Microbiol. Res. 165, 427–435 (2010).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013). A notable review on the mechanisms and the importance of immunogenic cell death in cancer.
Ellerby, H. M. et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 5, 1032–1038 (1999).
Arap, M. A. et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 6, 275–284 (2004).
Pasqualini, R. et al. Targeting the interleukin-11 receptor α in metastatic prostate cancer: a first-in-man study. Cancer 121, 2411–2421 (2015).
Paulos, C. M. et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204 (2007).
Galluzzi, L. et al. Trial Watch: experimental Toll-like receptor agonists for cancer therapy. Oncoimmunology 1, 699–716 (2012).
Paavonen, J. et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374, 301–314 (2009).
Walter, A. et al. Aldara activates TLR7-independent immune defence. Nat. Commun. 4, 1560 (2013).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Chatterjee, S. et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res. 74, 5008–5018 (2014).
Mehmeti, M. et al. Expression of functional toll like receptor 4 in estrogen receptor/progesterone receptor-negative breast cancer. Breast Cancer Res. 17, 130 (2015).
Martin, F.-P. J. et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol. Syst. Biol. 3, 112 (2007).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Perry, R. J. et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015).
Jan, G. et al. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 9, 179–188 (2002).
Wei, W., Sun, W., Yu, S., Yang, Y. & Ai, L. Butyrate production from high-fiber diet protects against lymphoma tumor. Leuk. Lymphoma 57, 2401–2408 (2016).
Kaiko, G. E. et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165, 1708–1720 (2016).
Belcheva, A. et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 158, 288–299 (2014).
Bultman, S. J. & Jobin, C. Microbial-derived butyrate: an oncometabolite or tumor-suppressive metabolite? Cell Host Microbe 16, 143–145 (2014).
Gutiérrez-Díaz, I., Fernández-Navarro, T., Sánchez, B., Margolles, A. & González, S. Mediterranean diet and faecal microbiota: a transversal study. Food Funct. 7, 2347–2356 (2016).
Mathewson, N. D. et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513 (2016).
Bui, T. P. N. et al. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 6, 10062 (2015).
Wahlström, A., Sayin, S. I., Marschall, H.-U. & Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Eisenberg, T. et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 1428–1438 (2016).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009). This paper shows that administration of the natural polyamine spermidine in ageing mice triggers epigenetic deacetylation of histone H3, decreasing oxidative stress and necrosis.
Matsumoto, M., Kurihara, S., Kibe, R., Ashida, H. & Benno, Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 6, e23652 (2011).
Magnúsdóttir, S., Ravcheev, D., de Crécy-Lagard, V. & Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 6, 148 (2015).
Galluzzi, L. et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2, 257–269 (2012).
Aranda, F. et al. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene 34, 3053–3062 (2015).
Commichau, F. M. et al. Engineering Bacillus subtilis for the conversion of the antimetabolite 4-hydroxy-l-threonine to pyridoxine. Metab. Eng. 29, 196–207 (2015).
Heimann, D. M. & Rosenberg, S. A. Continuous intravenous administration of live genetically modified Salmonella typhimurium in patients with metastatic melanoma. J. Immunother. 26, 179–180 (2003).
Bhattacharya, N. et al. Normalizing microbiota-induced retinoic acid deficiency stimulates protective CD8+ T cell-mediated immunity in colorectal cancer. Immunity 45, 641–655 (2016). This study shows that, in colon cancer, all- trans -retinoic acid supplementation has a key role by enhancing CD8+ T cell migration to the tumour microenvironment.
Smith, M. B., Kelly, C. & Alm, E. J. Policy: how to regulate faecal transplants. Nature 506, 290–291 (2014).
Sartor, R. B. Microbial influences in inflammatory bowel diseases. Gastroenterology 134, 577–594 (2008).
Zoetendal, E. G. et al. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl. Environ. Microbiol. 68, 3401–3407 (2002).
Ringel, Y. et al. High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes 6, 173–181 (2015).
Wexler, A. G. et al. Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc. Natl Acad. Sci. USA 113, 3639–3644 (2016).
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Rajagopala, S. V. et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent acute lymphoblastic leukemia (ALL) at the time of disease diagnosis. BMC Genomics 17, 635 (2016).
Fan, X. et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut http://dx.doi.org/10.1136/gutjnl-2016-312580 (2016).
Chng, K. R. et al. Tissue microbiome profiling identifies an enrichment of specific enteric bacteria in Opisthorchis viverrini associated cholangiocarcinoma. EBioMedicine 8, 195–202 (2016).
Murphy, G. et al. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology 60, 1963–1971 (2014).
Xu, W. et al. Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am. J. Clin. Exp. Urol. 2, 57–61 (2014).
Schmidt, B. L. et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 9, e98741 (2014).
Hosgood, H. D. et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ. Mol. Mutagen. 55, 643–651 (2014).
Martínez-Piñeiro, J. A. et al. Bacillus Calmette–Guerin versus doxorubicin versus thiotepa: a randomized prospective study in 202 patients with superficial bladder cancer. J. Urol. 143, 502–506 (1990).
Aso, Y. & Akazan, H. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urol. Int. 49, 125–129 (1992).
Ohashi, Y. et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol. Int. 68, 273–280 (2002).
Hoesl, C. E. & Altwein, J. E. The probiotic approach: an alternative treatment option in urology. Eur. Urol. 47, 288–296 (2005).
Stebbing, J. et al. An intra-patient placebo-controlled phase I trial to evaluate the safety and tolerability of intradermal IMM-101 in melanoma. Ann. Oncol. 23, 1314–1319 (2012).
Dalgleish, A. G. et al. Randomised, open-label, phase II study of gemcitabine with and without IMM-101 for advanced pancreatic cancer. Br. J. Cancer 115, 789–796 (2016).
Le, D. T. et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 33, 1325–1333 (2015).
Liu-Chittenden, Y. et al. Phase I trial of systemic intravenous infusion of interleukin-13-Pseudomonas exotoxin in patients with metastatic adrenocortical carcinoma. Cancer Med. 4, 1060–1068 (2015).
Weber, F. et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 64, 125–137 (2003).
Toso, J. F. et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 20, 142–152 (2002). This study shows that attenuated S . Typhimurium can be safely administered to patients with cancer and is able to colonize some tumours.
Nemunaitis, J. et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 10, 737–744 (2003).
Laske, D. W., Youle, R. J. & Oldfield, E. H. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat. Med. 3, 1362–1368 (1997).
Roberts, N. J. et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl Med. 6, 249ra111 (2014).
Kato, I., Kobayashi, S., Yokokura, T. & Mutai, M. Antitumor activity of Lactobacillus casei in mice. Gan 72, 517–523 (1981).
Tomita, K. et al. Influence of Lactobacillus casei on rat bladder carcinogenesis [Japanese]. Nihon Hinyokika Gakkai Zasshi 85, 655–663 (1994).
Takahashi, T. et al. Antitumor effects of the intravesical instillation of heat killed cells of the Lactobacillus casei strain Shirota on the murine orthotopic bladder tumor MBT-2. J. Urol. 166, 2506–2511 (2001).
Seow, S. W. et al. Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium bovis (bacillus Calmette-Guerin). J. Urol. 168, 2236–2239 (2002).
Seow, S. W., Rahmat, J. N., Bay, B. H., Lee, Y. K. & Mahendran, R. Expression of chemokine/cytokine genes and immune cell recruitment following the instillation of Mycobacterium bovis, bacillus Calmette-Guérin or Lactobacillus rhamnosus strain GG in the healthy murine bladder. Immunology 124, 419–427 (2008).
Takagi, A. et al. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 22, 599–605 (2001).
Urbanska, A. M., Bhathena, J., Martoni, C. & Prakash, S. Estimation of the potential antitumor activity of microencapsulated Lactobacillus acidophilus yogurt formulation in the attenuation of tumorigenesis in Apc(Min/+) mice. Dig. Dis. Sci. 54, 264–273 (2009).
Urbanska, A. M., Bhathena, J., Cherif, S. & Prakash, S. Orally delivered microencapsulated probiotic formulation favorably impacts polyp formation in APC (Min/+) model of intestinal carcinogenesis. Artif. Cells Nanomed. Biotechnol. 44, 1–11 (2016).
Le Leu, R. K., Hu, Y., Brown, I. L., Woodman, R. J. & Young, G. P. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis 31, 246–251 (2010).
Cheng, M. et al. Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism. Cancer Res. 74, 4030–4041 (2014). This study shows that microbiota modifications in antibiotic-treated mice decrease the induction of γδ T cells and influence immunosurveillance.
Ma, E. L. et al. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int. J. Cancer 127, 780–790 (2010).
Lan, A., Lagadic-Gossmann, D., Lemaire, C., Brenner, C. & Jan, G. Acidic extracellular pH shifts colorectal cancer cell death from apoptosis to necrosis upon exposure to propionate and acetate, major end-products of the human probiotic propionibacteria. Apoptosis 12, 573–591 (2007).
Thirabunyanon, M., Boonprasom, P. & Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 31, 571–576 (2009).
Strus, M. et al. Effect of hydrogen peroxide of bacterial origin on apoptosis and necrosis of gut mucosa epithelial cells as a possible pathomechanism of inflammatory bowel disease and cancer. J. Physiol. Pharmacol. 60 (Suppl. 6), 55–60 (2009).
Kim, Y., Oh, S., Yun, H. S., Oh, S. & Kim, S. H. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett. Appl. Microbiol. 51, 123–130 (2010).
Altonsy, M. O., Andrews, S. C. & Tuohy, K. M. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: mediation by the mitochondrial pathway. Int. J. Food Microbiol. 137, 190–203 (2010).
Acknowledgements
L.Z. and G.K. are supported by the Institut National Du Cancer (INCA), the Ligue contre le Cancer (équipe labelisée); Agence Nationale de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); the Paris Alliance of Cancer Research Institutes (PACRI); and the PIA2 TORINO-LUMIERE. L.Z. is also supported by the Swiss Institute for Experimental Cancer Research (ISREC), by the Swiss Bridge Foundation, and by IMMUNTRAIN-H2020. G.K. is also supported by the LeDucq Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
L.Z. and G.K. receive research support by Lytix Ltd and are co-founders of the biotechnological company EverImmune.
Glossary
- Microbiome
-
The collective genomes that can be found within a single microbial ecosystem.
- Microbiota
-
The community of microorganisms that exist within a single ecosystem.
- Metabolic syndrome
-
A syndrome characterized by central obesity, dyslipidaemia, increased blood pressure and high blood-sugar levels, increased risk of type 2 diabetes and cardiovascular disease.
- Immunosurveillance
-
A term that is used to describe the processes by which cells of the immune system hunt and target pathogens, such as bacteria and viruses, or pre-cancerous and cancerous host cells.
- Pattern recognition receptors
-
(PRRs). Innate immune components expressed by various cell types to sense infection or tissue damage.
- Toll-like receptors
-
Pattern recognition receptors that mostly recognize bacterial structures.
- Immune-checkpoint blockade
-
A pharmacological intervention whereby monoclonal antibodies neutralize major inhibitory receptors (such as cytotoxic T lymphocyte protein 4 (CTLA4) and programmed cell death 1 (PD1)) expressed by activated lymphocytes to alleviate immune suppression and restore lymphocyte effector functions.
- Faecal microbiota transplantation
-
The engraftment of microbiota from a healthy donor into a recipient, which results in the restoration of the normal gut microbial ecosystem.
- Graft-versus-host disease
-
An immune attack of transplanted lymphocytes against host cells, which causes systemic disease following the transfusion of cells from a donor that has distinct histocompatibility antigens.
- Probiotic
-
A live microorganism that can confer a health benefit to the host.
- Prebiotic
-
A non-digestible food ingredient that stimulates the growth and activity of bacteria in the digestive system.
- Autophagy
-
A mechanism of lysosomal degradation that enables the degradation and recycling of cytoplasmic material sequestered in autophagosomes.
- Sarcopenia
-
The degenerative loss of skeletal muscle mass, quality and strength associated with ageing, frailty syndrome and/or cachexia.
- Thymus atrophy
-
An age-dependent reduction in thymic mass that may be accelerated in pathological conditions.
- TH17 cell
-
(T helper 17 cell). A CD4+ T helper cell induced by the coordinated action of transforming growth factor-β (TGFβ) and interleukin-6 (IL-6), to activate the transcription factor retinoid-related orphan nuclear receptor-γt (RORγt) and to produce IL-17 and IL-22.
- Tr1 cells
-
(T regulatory type 1 cells) CD4+ T regulatory type 1 cells that produce large amounts of interleukin-10 (IL-10) through IL-10R signalling, and induce an anti-inflammatory response.
- Ectopic expression
-
Enforced expression of a gene product, triggered by somatic mutation or genetic manipulation.
- Intestinal crypts
-
Tube-like glands found in the lining of the colon and rectum.
Rights and permissions
About this article
Cite this article
Zitvogel, L., Daillère, R., Roberti, M. et al. Anticancer effects of the microbiome and its products. Nat Rev Microbiol 15, 465–478 (2017). https://doi.org/10.1038/nrmicro.2017.44
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2017.44
This article is cited by
-
Unlocking the secrets: exploring the influence of the aryl hydrocarbon receptor and microbiome on cancer development
Cellular & Molecular Biology Letters (2024)
-
Intratumoral microorganisms in tumors of the digestive system
Cell Communication and Signaling (2024)
-
Adipose tissue macrophages: implications for obesity-associated cancer
Military Medical Research (2023)
-
Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications
Signal Transduction and Targeted Therapy (2023)
-
Comparison of DNA extraction methods for 16S rRNA gene sequencing in the analysis of the human gut microbiome
Scientific Reports (2023)