Key Points
-
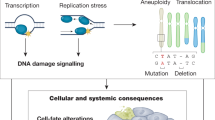
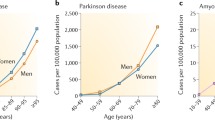
The world's most common diseases are chronic ageing-associated illnesses.
-
Premature ageing diseases and ageing-associated diseases (AADs) share the common hallmarks of increased genomic instability, altered metabolic signalling and reduced regenerative potency.
-
Premature ageing diseases are powerful models to study the cellular and molecular causes and mechanisms of physiological ageing and AADs.
-
Decreased efficiency of DNA repair, shortening of telomeres and loss of heterochromatin underlie the loss of genomic integrity in (premature) ageing and AADs.
-
Increased metabolic signalling contributes to increased levels of oxidative stress, which compromise the integrity of the cellular genome and proteome in ageing.
-
Chronic stress permanently alters cellular fate by inducing senescence and reducing the regenerative capacity of stem cells, which ultimately drives physiological decline in ageing.
-
Segmental progeroid syndromes provide exciting new opportunities to understand and therapeutically correct the loss of cellular homeostasis in ageing and disease.
Abstract
Ageing is the predominant risk factor for many common diseases. Human premature ageing diseases are powerful model systems to identify and characterize cellular mechanisms that underpin physiological ageing. Their study also leads to a better understanding of the causes, drivers and potential therapeutic strategies of common diseases associated with ageing, including neurological disorders, diabetes, cardiovascular diseases and cancer. Using the rare premature ageing disorder Hutchinson–Gilford progeria syndrome as a paradigm, we discuss here the shared mechanisms between premature ageing and ageing-associated diseases, including defects in genetic, epigenetic and metabolic pathways; mitochondrial and protein homeostasis; cell cycle; and stem cell-regenerative capacity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lopez, A. D., Mathers, C. D., Ezzati, M., Jamison, D. T. & Murray, C. J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367, 1747–1757 (2006).
National Center for Health Statistics. Deaths and mortality. Center for Disease Control and Prevention http://www.cdc.gov/nchs/fastats/deaths.htm (2017).
Denayer, T., Stöhr, T. & Van Roy, M. Animal models in translational medicine: validation and prediction. New Horizons Transl Med. 2, 5–11 (2014).
Potashkin, J. A., Blume, S. R. & Runkle, N. K. Limitations of animal models of Parkinson's disease. Parkinsons Dis. 2011, 658083 (2010).
Getz, G. S. & Reardon, C. A. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 1104–1115 (2012).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016). An important study elegantly demonstrating the detrimental effect of the accumulation of senescent cells on lifespan and healthspan.
Nystrom, H., Nordstrom, A. & Nordstrom, P. Risk of injurious fall and hip fracture up to 26 y before the diagnosis of Parkinson disease: nested case-control studies in a nationwide cohort. PLoS Med. 13, e1001954 (2016).
Chang, Y. T. et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol. Dial. Transplant. 26, 3588–3595 (2011).
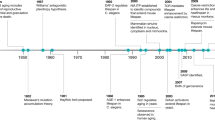
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 423, 293–298 (2003).
de Sandre-Giovannoli, A. et al. Lamin A truncation in Hutchinson–Gilford progeria. Science 1084, 2055 (2003).
Yu, C. E. et al. Positional cloning of the Werner's syndrome gene. Science 272, 258–262 (1996).
Hennekam, R. C. Hutchinson–Gilford progeria syndrome: review of the phenotype. Am. J. Med. Genet. A 140, 2603–2624 (2006).
Gordon, L. B. et al. Impact of farnesylation inhibitors on survival in Hutchinson–Gilford progeria syndrome. Circulation 130, 27–34 (2014).
Gordon, L. B., Rothman, F. G., Lopez-Otin, C. & Misteli, T. Progeria: a paradigm for translational medicine. Cell 156, 400–407 (2014).
Dittmer, T. A. & Misteli, T. The lamin protein family. Genome Biol. 12, 222 (2011).
Davies, B. S., Fong, L. G., Yang, S. H., Coffinier, C. & Young, S. G. The posttranslational processing of prelamin A and disease. Annu. Rev. Genomics Hum. Genet. 10, 153–174 (2009).
Taimen, P. et al. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc. Natl Acad. Sci. USA 106, 20788–20793 (2009).
Dahl, K. N., Ribeiro, A. J. & Lammerding, J. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 102, 1307–1318 (2008).
Carrero, D. & Soria-Valles, C. Hallmarks of progeroid syndromes: lessons from mice and reprogrammed cells. Dis. Model. Mech. 9, 719–735 (2016).
de la Rosa, J. et al. Prelamin A causes progeria through cell-extrinsic mechanisms and prevents cancer invasion. Nat. Commun. 4, 2268 (2013).
Jung, H. J. et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc. Natl Acad. Sci. USA 109, E423–E431 (2012).
Fernandez, P. et al. Transformation resistance in a premature aging disorder identifies a tumor-protective function of BRD4. Cell Rep. 9, 248–260 (2014).
Merideth, M. A. et al. Phenotype and course of Hutchinson–Gilford progeria syndrome. N. Engl. J. Med. 358, 592–604 (2008).
Broers, J. L., Ramaekers, F. C., Bonne, G., Yaou, R. B. & Hutchison, C. J. Nuclear lamins: laminopathies and their role in premature ageing. Physiol. Rev. 86, 967–1008 (2006).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013). A comprehensive overview of the cellular defects that occur during ageing.
Kubben, N. et al. Repression of the antioxidant Nrf2 pathway in premature aging. Cell 165, 1361–1374 (2016). An important study that used unbiased screening to identify the mechanisms that drive premature cellular ageing. Entrapment of the antioxidant-promoting transcription factor NRF2 by progerin was found as a novel mechanism that drives HGPS and is likely a cause of chronic oxidative stress during ageing.
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006). This study provided the first evidence that similar changes in histone modifications occur in HGPS and normal ageing.
Viteri, G., Chung, Y. W. & Stadtman, E. R. Effect of progerin on the accumulation of oxidized proteins in fibroblasts from Hutchinson Gilford progeria patients. Mech. Ageing Dev. 131, 2–8 (2010).
Liu, G. H. et al. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 472, 221–225 (2011).
Zhang, J. et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8, 31–45 (2011).
Pegoraro, G. et al. Ageing-related chromatin defects through loss of the NURD complex. Nat. Cell Biol. 11, 1261–1267 (2009).
Van Berlo, J. H. et al. A-Type lamins are essential for TGF-beta1 induced PP2A to dephosphorylate transcription factors. Hum. Mol. Genet. 14, 2839–2849 (2005).
Varela, I. et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437, 564–568 (2005). This study provided important proof that chronic activation of the p53 pathway as a response to elevated cellular stress has a detrimental effect in prelamin A-induced premature ageing.
Puente, X. S. et al. Exome sequencing and functional analysis identifies BANF1 mutation as the cause of a hereditary progeroid syndrome. Am. J. Hum. Genet. 88, 650–656 (2011).
McClintock, D. et al. The mutant form of lamin A that causes Hutchinson–Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE 2, e1269 (2007).
Lopez-Mejia, I. C. et al. Antagonistic functions of LMNA isoforms in energy expenditure and lifespan. EMBO Rep. 15, 529–539 (2014).
Hisama, F. M. et al. Coronary artery disease in a Werner syndrome-like form of progeria characterized by low levels of progerin, a splice variant of lamin A. Am J Med Genet A 155, 3002–3006 (2011).
Atamna, H., Cheung, I. & Ames, B. N. A method for detecting abasic sites in living cells: age-dependent changes in base excision repair. Proc. Natl Acad. Sci. USA 97, 686–691 (2000).
Annett, K. et al. An investigation of DNA mismatch repair capacity under normal culture conditions and under conditions of supra-physiological challenge in human CD4+T cell clones from donors of different ages. Exp. Gerontol. 40, 976–981 (2005).
Moriwaki, S., Ray, S., Tarone, R. E., Kraemer, K. H. & Grossman, L. The effect of donor age on the processing of UV-damaged DNA by cultured human cells: reduced DNA repair capacity and increased DNA mutability. Mutat. Res. 364, 117–123 (1996).
Mao, Z. et al. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc. Natl Acad. Sci. USA 109, 11800–11805 (2012).
Stuart, G. R. & Glickman, B. W. Through a glass, darkly: reflections of mutation from lacI transgenic mice. Genetics 155, 1359–1367 (2000).
Liu, Y. et al. Involvement of xeroderma pigmentosum group A (XPA) in progeria arising from defective maturation of prelamin A. FASEB J. 22, 603–611 (2008). This paper provides a mechanistic explanation for the frequently observed persistence of irreparable DNA damage in HGPS.
Musich, P. R. & Zou, Y. Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Aging (Albany NY) 1, 28–37 (2009).
Tang, H., Hilton, B., Musich, P. R., Fang, D. Z. & Zou, Y. Replication factor C1, the large subunit of replication factor C, is proteolytically truncated in Hutchinson–Gilford progeria syndrome. Aging Cell 11, 363–365 (2012).
Cobb, A. M., Murray, T. V., Warren, D. T., Liu, Y. & Shanahan, C. M. Disruption of PCNA-lamins A/C interactions by prelamin A induces DNA replication fork stalling. Nucleus 7, 498–511 (2016).
Zhang, H., Xiong, Z. M. & Cao, K. Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly(ADP-ribose) polymerase 1. Proc. Natl Acad. Sci. USA 111, E2261–E2270 (2014).
Liu, B. et al. Genomic instability in laminopathy-based premature aging. Nat. Med. 11, 780–785 (2005).
Rass, U., Ahel, I. & West, S. C. Defective DNA repair and neurodegenerative disease. Cell 130, 991–1004 (2007).
Shackelford, D. A. DNA end joining activity is reduced in Alzheimer's disease. Neurobiol. Aging 27, 596–605 (2006).
Kao, S. Y. Regulation of DNA repair by parkin. Biochem. Biophys. Res. Commun. 382, 321–325 (2009).
Robbins, J. H. et al. Parkinson's disease and Alzheimer's disease: hypersensitivity to X rays in cultured cell lines. J. Neurol. Neurosurg. Psychiatry 48, 916–923 (1985).
Mahmoudi, M., Mercer, J. & Bennett, M. DNA damage and repair in atherosclerosis. Cardiovasc. Res. 71, 259–268 (2006).
Muftuoglu, M. et al. The clinical characteristics of Werner syndrome: molecular and biochemical diagnosis. Hum. Genet. 124, 369–377 (2008).
Tavana, O. et al. Ku70 functions in addition to nonhomologous end joining in pancreatic beta-cells: a connection to beta-catenin regulation. Diabetes 62, 2429–2438 (2013).
Schmidt, E. et al. Expression of the Hutchinson–Gilford progeria mutation during osteoblast development results in loss of osteocytes, irregular mineralization, and poor biomechanical properties. J. Biol. Chem. 287, 33512–33522 (2012).
Lombard, D. B. et al. DNA repair, genome stability, and aging. Cell 120, 497–512 (2005).
Gorbunova, V., Seluanov, A., Mao, Z. & Hine, C. Changes in DNA repair during aging. Nucleic Acids Res. 35, 7466–7474 (2007).
Fuster, J. J. & Andres, V. Telomere biology and cardiovascular disease. Circ. Res. 99, 1167–1180 (2006).
Wong, L. S. et al. Telomere biology in cardiovascular disease: the TERC−/− mouse as a model for heart failure and ageing. Cardiovasc. Res. 81, 244–252 (2009).
Benson, E. K., Lee, S. W. & Aaronson, S. A. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J. Cell Sci. 123, 2605–2612 (2010).
Decker, M. L., Chavez, E., Vulto, I. & Lansdorp, P. M. Telomere length in Hutchinson–Gilford progeria syndrome. Mech. Ageing Dev. 130, 377–383 (2009).
Gonzalez-Suarez, I. et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 28, 2414–2427 (2009).
De Vos, W. H. et al. Increased plasticity of the nuclear envelope and hypermobility of telomeres due to the loss of A-type lamins. Biochim. Biophys. Acta 1800, 448–458 (2010).
Raz, V. et al. The nuclear lamina promotes telomere aggregation and centromere peripheral localization during senescence of human mesenchymal stem cells. J. Cell Sci. 121, 4018–4028 (2008).
Wood, A. M. et al. TRF2 and lamin A/C interact to facilitate the functional organization of chromosome ends. Nat. Commun. 5, 5467 (2014).
Saha, B. et al. DNA damage accumulation and TRF2 degradation in atypical Werner syndrome fibroblasts with LMNA mutations. Front. Genet. 4, 129 (2013).
Cao, K. et al. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Invest. 121, 2833–2844 (2011).
Li, D., Yuan, Q. & Wang, W. The role of telomeres in musculoskeletal diseases. J. Int. Med. Res. 40, 1242–1250 (2012).
Kordinas, V., Ioannidis, A. & Chatzipanagiotou, S. The telomere/telomerase system in chronic inflammatory diseases. Cause or effect? Genes (Basel) 7, 60 (2016).
Alder, J. K. et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl Acad. Sci. USA 105, 13051–13056 (2008).
Adnot, S. et al. Telomere dysfunction and cell senescence in chronic lung diseases: therapeutic potential. Pharmacol. Ther. 153, 125–134 (2015).
Chojnowski, A. et al. Progerin reduces LAP2alpha-telomere association in Hutchinson–Gilford progeria. eLife 4, e07759 (2015).
Heyn, H., Moran, S. & Esteller, M. Aberrant DNA methylation profiles in the premature aging disorders Hutchinson–Gilford Progeria and Werner syndrome. Epigenetics 8, 28–33 (2013).
Scaffidi, P. & Misteli, T. Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nat. Med. 11, 440–445 (2005).
Shumaker, D. K. et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl Acad. Sci. USA 103, 8703–8708 (2006).
Jin, C. et al. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab. 14, 161–172 (2011).
Chen, H. et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 23, 975–985 (2009).
Lund, H. L. & van Loohuizen, M. Polycomb complexes and silencing mechanisms. Curr. Opin. Cell Biol. 16, 239–246 (2004).
Marullo, F. et al. Nucleoplasmic Lamin A/C and Polycomb group of proteins: an evolutionarily conserved interplay. Nucleus 7, 103–111 (2016).
Zhang, W. et al. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348, 1160–1163 (2015). This paper demonstrates that global epigenetic defects underlie mesenchymal stem cell dysfunction in Werner premature ageing syndrome.
Liu, B. et al. Depleting the methyltransferase Suv39h1 improves DNA repair and extends lifespan in a progeria mouse model. Nat. Commun. 4, 1868 (2013).
Wing, M. R., Ramezani, A., Gill, H. S., Devaney, J. M. & Raj, D. S. Epigenetics of progression of chronic kidney disease: fact or fantasy? Semin. Nephrol. 33, 363–374 (2013).
Frost, B., Hemberg, M., Lewis, J. & Feany, M. B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 17, 357–366 (2014).
Misteli, T. Higher-order genome organization in human disease. Cold Spring Harb. Perspect. Biol. 2, a000794 (2010).
Krishnan, V. et al. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24- deficient mice. Proc. Natl Acad. Sci. USA 108, 12325–12330 (2011).
Sharma, G. G. et al. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol. Cell. Biol. 30, 3582–3595 (2010).
Dang, W. et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459, 802–807 (2009).
Peleg, S. et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328, 753–756 (2010).
Qiu, X., Xiao, X., Li, N. & Li, Y. Histone deacetylases inhibitors (HDACis) as novel therapeutic application in various clinical diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry 72, 60–72 (2017).
Mazucanti, C. H. et al. Longevity pathways (mTOR, SIRT, Insulin/IGF-1) as key modulatory targets on aging and neurodegeneration. Curr. Top. Med. Chem. 15, 2116–2138 (2015).
Mariño, G. et al. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl Acad. Sci. USA 107, 16268–16273 (2010).
Perluigi, M., Di Domenico, F. & Butterfield, D. A. mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol. Dis. 84, 39–49 (2015).
Talaei, F., van Praag, V. M. & Henning, R. H. Hydrogen sulfide restores a normal morphological phenotype in Werner syndrome fibroblasts, attenuates oxidative damage and modulates mTOR pathway. Pharmacol. Res. 74, 34–44 (2013).
Sharples, A. P. et al. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 14, 511–523 (2015).
Massudi, H. et al. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE 7, e42357 (2012).
Liu, B. et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 16, 738–750 (2012).
Jeon, S. M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 48, e245 (2016).
Satriano, J., Sharma, K., Blantz, R. C. & Deng, A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am. J. Physiol. Renal Physiol. 305, F727–F733 (2013).
Anderson, J. G. et al. Enhanced insulin sensitivity in skeletal muscle and liver by physiological overexpression of SIRT6. Mol. Metab. 4, 846–856 (2015).
Endisha, H. et al. Restoring SIRT6 expression in Hutchinson–Gilford progeria syndrome cells impedes premature senescence and formation of dysmorphic nuclei. Pathobiology 82, 9–20 (2015).
Lane, R. K., Hilsabeck, T. & Rea, S. L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta 1847, 1387–1400 (2015).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004). This study provided direct evidence that the accumulation of mutations in mtDNA that occurs with ageing is not simply correlative but causes a eduction in lifespan and leads to the development of premature ageing pathologies.
Xiong, Z. M. et al. Methylene blue alleviates nuclear and mitochondrial abnormalities in progeria. Aging Cell 15, 279–290 (2015).
Rivera-Torres, J. et al. Identification of mitochondrial dysfunction in Hutchinson–Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture. J. Proteomics 91, 466–477 (2013).
Massip, L. et al. Vitamin C restores healthy aging in a mouse model for Werner syndrome. FASEB J. 24, 158–172 (2010).
Jiang, T., Sun, Q. & Chen, S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease. Prog. Neurobiol. 147, 1–19 (2016).
de Souza-Pinto, N. C., Wilson, D. M. III, Stevnsner, T. V. & Bohr, V. A. Mitochondrial DNA, base excision repair and neurodegeneration. DNA Repair (Amst.) 7, 1098–1109 (2008).
Golpich, M. et al. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci. Ther. 23, 5–22 (2016).
Kang, M. J. & Shadel, G. S. A mitochondrial perspective of chronic obstructive pulmonary disease pathogenesis. Tuberc. Respir. Dis. (Seoul) 79, 207–213 (2016).
Villa-Bellosta, R. et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson–Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 127, 2442–2451 (2013).
Demer, L. L. & Tintut, Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117, 2938–2948 (2008).
Dinkova-Kostova, A. T. & Abramov, A. Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 88, 179–188 (2015).
Pallardo, F. V. et al. Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia–Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology 11, 401–419 (2010).
Suh, J. H. et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl Acad. Sci. USA 101, 3381–3386 (2004).
Ramsey, C. P. et al. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 66, 75–85 (2007).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005). This paper provides evidence that mitochondria are a prominent source of oxidative stress that drives ageing.
Tamaki, M. et al. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int. 85, 1330–1339 (2014).
Tanaka, K. & Matsuda, N. Proteostasis and neurodegeneration: the roles of proteasomal degradation and autophagy. Biochim. Biophys. Acta 1843, 197–204 (2014).
Cao, K. et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson–Gilford progeria syndrome cells. Sci. Transl Med. 3, 89ra58 (2011). This paper provides a mechanistic link between metabolic signalling and ageing via the autophagic degradation of progerin and offers a novel therapeutic strategy for HGPS that is currently being tested in a clinical trial.
Anisimov, V. N. et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10, 4230–4236 (2011).
Di Domenico, F., Tramutola, A. & Perluigi, M. Cathepsin D as a therapeutic target in Alzheimer's disease. Expert Opin. Ther. Targets 20, 1393–1395 (2016).
Caccamo, A., De Pinto, V., Messina, A., Branca, C. & Oddo, S. Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer's disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J. Neurosci. 34, 7988–7998 (2014).
Xilouri, M. et al. Impairment of chaperone-mediated autophagy induces dopaminergic neurodegeneration in rats. Autophagy 12, 2230–2247 (2016).
Wang, X. & Robbins, J. Proteasomal and lysosomal protein degradation and heart disease. J. Mol. Cell. Cardiol. 71, 16–24 (2014).
Tai, S., Hu, X. Q., Peng, D. Q., Zhou, S. H. & Zheng, X. L. The roles of autophagy in vascular smooth muscle cells. Int. J. Cardiol. 211, 1–6 (2016).
Mukherjee, A., Morales-Scheihing, D., Butler, P. C. & Soto, C. Type 2 diabetes as a protein misfolding disease. Trends Mol. Med. 21, 439–449 (2015). An interesting perspective on T2D, which has joined the growing ranks of diseases related to protein misfolding.
Saez, I. & Vilchez, D. The mechanistic links between proteasome activity, aging and age-related diseases. Curr. Genomics 15, 38–51 (2014).
Deger, J. M., Gerson, J. E. & Kayed, R. The interrelationship of proteasome impairment and oligomeric intermediates in neurodegeneration. Aging Cell 14, 715–724 (2015).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Osorio, F. G. et al. Nuclear lamina defects cause ATM-dependent NF-kappaB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 26, 2311–2324 (2012). An important study depicting for the first time that chronic activation of inflammatory pathways is crucial for the formation of premature ageing defects in HGPS.
Ibrahim, M. X. et al. Targeting isoprenylcysteine methylation ameliorates disease in a mouse model of progeria. Science 340, 1330–1333 (2013).
Soria-Valles, C. et al. NF-kappaB activation impairs somatic cell reprogramming in ageing. Nat. Cell Biol. 17, 1004–1013 (2015).
Bhat, R. et al. Astrocyte senescence as a component of Alzheimer's disease. PLoS ONE 7, e45069 (2012).
Shi, Z. M. et al. Upstream regulators and downstream effectors of NF-kappaB in Alzheimer's disease. J. Neurol. Sci. 366, 127–134 (2016).
Phani, S., Loike, J. D. & Przedborski, S. Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism Relat. Disord. 18 (Suppl. 1), S207–S209 (2012).
Kuwano, K. et al. Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). Respir. Investig. 54, 397–406 (2016).
Decleves, A. E. & Sharma, K. Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat. Rev. Nephrol. 10, 257–267 (2014).
Iyemere, V. P., Proudfoot, D., Weissberg, P. L. & Shanahan, C. M. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J. Intern. Med. 260, 192–210 (2006).
Turinetto, V., Vitale, E. & Giachino, C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 17, E1164 (2016).
Rosengardten, Y., McKenna, T., Grochova, D. & Eriksson, M. Stem cell depletion in Hutchinson–Gilford progeria syndrome. Aging Cell 10, 1011–1020 (2011).
Scaffidi, P. & Misteli, T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat. Cell Biol. 10, 452–459 (2008).
Olive, M. et al. Cardiovascular pathology in Hutchinson–Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 30, 2301–2309 (2010).
Oh, J., Lee, Y. D. & Wagers, A. J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20, 870–880 (2014).
Bhartiya, D. & Patel, H. Very small embryonic-like stem cells are involved in pancreatic regeneration and their dysfunction with age may lead to diabetes and cancer. Stem Cell Res. Ther. 6, 96 (2015).
Navarro, S. & Driscoll, B. Regeneration of the aging lung: a mini-review. Gerontology 63, 270–280 (2016).
Wang, J. et al. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 132, 1909–1919 (2015).
Tincer, G., Mashkaryan, V., Bhattarai, P. & Kizil, C. Neural stem/progenitor cells in Alzheimer's disease. Yale J. Biol. Med. 89, 23–35 (2016).
Ocampo, A. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719–1733.e12 (2016).
Author information
Authors and Affiliations
Contributions
N. K. and T. M. researched data for the article, contributed to discussion of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Nucleotide excision repair
-
(NER). A DNA repair pathway specialized in the removal of bulky DNA adducts, including ultraviolet damage-induced thymidine dimers.
- Non-homologous end joining
-
(NHEJ). A DNA repair pathway that repairs double-strand breaks through direct ligation of the broken ends without the need of a homologous template.
- Oxidative phosphorylation
-
(OXPHOS). A process by which electrons are transferred from electron donors to electron acceptors, thereby releasing energy in the form of ATP. In prokaryotes, this process takes place in the inner mitochondrial membrane at the site of the electron transport chain.
- Ubiquitin–proteasome system
-
(UPS). A system that degrades proteins marked by degradation-specific ubiquitin marks in an ATP-dependent manner.
- Epithelial-to-mesenchymal transition
-
(EMT). A process by which epithelial cells undergo various molecular changes related to cell–cell adhesion, polarity and invasive properties, in order to become mesenchymal cells. EMT has beneficial roles in wound healing but exerts detrimental effects in organ fibrosis and the initiation of tumour metastasis.
Rights and permissions
About this article
Cite this article
Kubben, N., Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol 18, 595–609 (2017). https://doi.org/10.1038/nrm.2017.68
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2017.68
This article is cited by
-
Long-term variability in physiological measures in relation to mortality and epigenetic aging: prospective studies in the USA and China
BMC Medicine (2023)
-
Association of frailty with the incidence risk of cardiovascular disease and type 2 diabetes mellitus in long-term cancer survivors: a prospective cohort study
BMC Medicine (2023)
-
Associations of sleeping, sedentary and physical activity with phenotypic age acceleration: a cross-sectional isotemporal substitution model
BMC Geriatrics (2023)
-
Tetrahydroberberrubine retards heart aging in mice by promoting PHB2-mediated mitophagy
Acta Pharmacologica Sinica (2023)
-
Accelerated aging in articular cartilage by ZMPSTE24 deficiency leads to osteoarthritis with impaired metabolic signaling and epigenetic regulation
Cell Death & Disease (2023)