Key Points
-
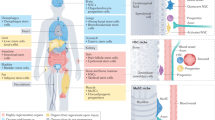
The concept of a strictly hierarchical model of tissue regeneration in which a uniform pool of stem cells that continuously regenerates somatic tissues has recently been re-evaluated.
-
In the haematopoietic system, stem cells with distinct differentiation preferences generate progeny in a lineage-biased manner. New data suggest that there is sustained production of specific cell types from highly specialized long-term progenitors, overturning traditional views.
-
The skin epithelium, which includes the interfollicular epidermis, the hair follicle and the sebaceous gland, contains heterogeneous pools of stem cells that maintain their discrete compartments during homeostasis. In transplantation assays or in response to injury, these stem cells are no longer restricted in their lineage choice.
-
Specific niches that surround the different pools of stem cells define the identities and behaviours of the stem cells.
-
Under normal conditions, the intestinal epithelium is primarily maintained by crypt base columnar (CBC) stem cells. Following injury, or in the absence of CBC cells, other cells can maintain and repair the intestine. Reserve or quiescent stem cells (qISCs; also known as +4 cells) are thought to be important for repair, but progenitors and differentiated cells may also revert to function as stem cells.
-
The identity and characteristics of stem cell types and the mechanisms of their differential regulation through intrinsic and extrinsic signals remain to be determined.
Abstract
Somatic stem cells replenish many tissues throughout life to repair damage and to maintain tissue homeostasis. Stem cell function is frequently described as following a hierarchical model in which a single master cell undergoes self-renewal and differentiation into multiple cell types and is responsible for most regenerative activity. However, recent data from studies on blood, skin and intestinal epithelium all point to the concomitant action of multiple types of stem cells with distinct everyday roles. Under stress conditions such as acute injury, the surprising developmental flexibility of these stem cells enables them to adapt to diverse roles and to acquire different regeneration capabilities. This paradigm shift raises many new questions about the developmental origins, inter-relationships and molecular regulation of these multiple stem cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pappenheim, A. Ueber Entwickelung und Ausbildung der Erythroblasten. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 145, 587–643 (in German) (1896).
Ramalho-Santos, M. & Willenbring, H. On the origin of the term “stem cell”. Cell Stem Cell 1, 35–38 (2007).
Becker, A. J., McCulloch, E. A. & Till, J. E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197, 452–454 (1963).
Lemischka, I. R., Raulet, D. H. & Mulligan, R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell 45, 917–927 (1986).
Keller, G. & Snodgrass, R. Life span of multipotential hematopoietic stem cells in vivo. J. Exp. Med. 171, 1407–1418 (1990).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine V. Unitarian theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 (1974).
Stange, D. E. et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368 (2013).
Van Keymeulen, A. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–193 (2011).
Ousset, M. et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nature Cell Biol. 14, 1131–1138 (2012).
Osawa, M., Hanada, K., Hamada, H. & Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242–245 (1996).
Müller-Sieburg, C. E., Cho, R. H., Thoman, M., Adkins, B. & Sieburg, H. B. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood 100, 1302–1309 (2002).
Muller-Sieburg, C. E., Cho, R. H., Karlsson, L., Huang, J. F. & Sieburg, H. B. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood 103, 4111–4118 (2004).
Dykstra, B. et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1, 218–229 (2007). This landmark study used single-cell transplantation to definitively establish various types of HSC with distinct differentiation preferences.
Benz, C. et al. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell 10, 273–283 (2012).
Cavazzana-Calvo, M. et al. Is normal hematopoiesis maintained solely by long-term multipotent stem cells? Blood 117, 4420–4424 (2011).
Challen, G. A., Boles, N. C., Chambers, S. M. & Goodell, M. A. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-ß1. Cell Stem Cell 6, 265–278 (2010). This study was the first to prospectively isolate HSC subtypes and show their differential regulation by a growth factor.
Morita, Y., Ema, H. & Nakauchi, H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med. 207, 1173–1182 (2010).
Beerman, I. et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107, 5465–5470 (2010).
Kent, D. G. et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood 113, 6342–6350 (2009).
Benveniste, P. et al. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell 6, 48–58 (2010).
Gekas, C. & Graf, T. CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood 121, 4463–4472 (2013).
Sanjuan-Pla, A. et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 502, 232–236 (2013).
Mallaney, C., Kothari, A., Martens, A. & Challen, G. A. Clonal-level responses of functionally distinct hematopoietic stem cells to trophic factors. Exp. Hematol. 42, 317–327. e2 (2014).
Wang, J. et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell 148, 1001–1014 (2012).
Ema, H., Morita, Y. & Suda, T. Heterogeneity and hierarchy of hematopoietic stem cells. Exp. Hematol. 42, 74–82. e2 (2014).
Ramos, C. A. et al. Evidence for diversity in transcriptional profiles of single hematopoietic stem cells. PLoS Genet. 2, e159 (2006).
Moignard, V. et al. Characterization of transcriptional networks in blood stem and progenitor cells using high-throughput single-cell gene expression analysis. Nature Cell Biol. 15, 363–372 (2013).
Guo, G. et al. Mapping cellular hierarchy by single-cell analysis of the cell surface repertoire. Cell Stem Cell 13, 492–505 (2013).
Cabezas-Wallscheid, N. et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell 15, 507–522 (2014).
Sun, D. et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 14, 673–688 (2014).
Beerman, I. et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 12, 413–425 (2013).
Challen, G. A. et al. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell 15, 350–364 (2014).
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 (2011).
Bruns, I. et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nature Med. 20, 1315–1320 (2014).
Scadden, D. T. Nice neighborhood: emerging concepts of the stem cell niche. Cell 157, 41–50 (2014).
Sun, J. et al. Clonal dynamics of native hematopoiesis. Nature 514, 322–327 (2014). This work uses transposon tagging to track haematopoietic differentiation in an unperturbed system in the absence of injury for the first time, showing that very long-lived progenitors can sustain a specific lineage over time.
Yamamoto, R. et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154, 1112–1126 (2013).
Adolfsson, J. et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121, 295–306 (2005).
Blanpain, C. & Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22, 339–373 (2006).
Müller-Rover, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3–15 (2001).
Rompolas, P. et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487, 496–499 (2012).
Chi, W., Wu, E. & Morgan, B. A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 140, 1676–1683 (2013).
Kobayashi, K., Rochat, A. & Barrandon, Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc. Natl Acad. Sci. USA 90, 7391–7395 (1993).
Morris, R. J. & Potten, C. S. Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif. 27, 279–289 (1994).
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Trempus, C. S. et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511 (2003).
Horsley, V., Aliprantis, A. O., Polak, L., Glimcher, L. H. & Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310 (2008).
Liu, Y., Lyle, S., Yang, Z. & Cotsarelis, G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Invest. Dermatol. 121, 963–968 (2003).
Youssef, K. K. et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nature Cell Biol. 12, 299–305 (2010).
DasGupta, R. & Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 (1999).
Nguyen, H. et al. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nature Genet. 41, 1068–1075 (2009).
Rhee, H., Polak, L. & Fuchs, E. Lhx2 maintains stem cell character in hair follicles. Science 312, 1946–1949 (2006).
Nowak, J. A., Polak, L., Pasolli, H. A. & Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3, 33–43 (2008).
Jaks, V. et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genet. 40, 1291–1299 (2008).
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Morris, R. J. et al. Capturing and profiling adult hair follicle stem cells. Nature Biotech. 22, 411–417 (2004).
Claudinot, S., Nicolas, M., Oshima, H., Rochat, A. & Barrandon, Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl Acad. Sci. USA 102, 14677–14682 (2005).
Snippert, H. J. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010).
Jensen, U. B. et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell Sci. 121, 609–617 (2008).
Nijhof, J. G. et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133, 3027–3037 (2006).
Jensen, K. B. et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4, 427–439 (2009).
Ghazizadeh, S. & Taichman, L. B. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20, 1215–1222 (2001).
Levy, V., Lindon, C., Harfe, B. D. & Morgan, B. A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 9, 855–861 (2005).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 11, 1351–1354 (2005).
Clayton, E. et al. A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 (2007).
Mascre, G. et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489, 257–262 (2012).
Page, M. E., Lombard, P., Ng, F., Göttgens, B. & Jensen, K. B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482 (2013). Using lineage tracing, this paper shows that discrete pools of stem cells contribute to distinct compartments of the skin epithelium.
Brownell, I., Guevara, E., Bai, C. B., Loomis, C. A. & Joyner, A. L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552–565 (2011).
Howard, J. M., Nuguid, J. M., Ngole, D. & Nguyen, H. Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development 141, 3143–3152 (2014).
Horsley, V. et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126, 597–609 (2006).
Kretzschmar, K. et al. BLIMP1 is required for postnatal epidermal homeostasis but does not define a sebaceous gland progenitor under steady-state conditions. Stem Cell Rep. 3, 620–633 (2014).
Liao, X. H. & Nguyen, H. Epidermal expression of Lgr6is dependent on nerve endings and Schwann cells. Exp. Dermatol. 23, 195–198 (2014).
Hsu, Y. C., Pasolli, H. A. & Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92–105 (2011).
Rompolas, P., Mesa, K. R. & Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518 (2013). Using laser ablation and striking live imaging, this paper shows that the niche determines the fate of stem cells.
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009).
Levy, V., Lindon, C., Zheng, Y., Harfe, B. D. & Morgan, B. A. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 21, 1358–1366 (2007).
Festa, E. et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771 (2011).
Fujiwara, H. et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589 (2011).
Nishimura, E. K. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–860 (2002).
Chang, C. Y. et al. NFIB is a governor of epithelial–melanocyte stem cell behaviour in a shared niche. Nature 495, 98–102 (2013).
Mezoff, E. & Shroyer, N. in Pediatric Gastrointestinal and Liver Diseases 5th edn (eds Hyams, J. S. & Wylie, R.) 324–336 (Elsevier, 2014).
Potten, C. S., Hume, W. J., Reid, P. & Cairns, J. The segregation of DNA in epithelial stem cells. Cell 15, 899–906 (1978).
Potten, C. S., Owen, G. & Booth, D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115, 2381–2388 (2002).
Gracz, A. D. & Magness, S. T. Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G260–G273 (2014).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
van der Flier, L. G. et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136, 903–912 (2009).
Munoz, J. et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. EMBO J. 31, 3079–3091 (2012).
Fafilek, B. et al. Troy, a tumor necrosis factor receptor family member, interacts with Lgr5 to inhibit Wnt signaling in intestinal stem cells. Gastroenterology 144, 381–391 (2013).
Schuijers, J., van der Flier, L. G., van Es, J. & Clevers, H. Robust Cre-mediated recombination in small intestinal stem cells utilizing the Olfm4 locus. Stem Cell Rep. 3, 234–241 (2014).
Li, L. & Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010).
Sangiorgi, E. & Capecchi, M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nature Genet. 40, 915–920 (2008).
Westphalen, C. B. et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Invest. 124, 1283–1295 (2014).
Takeda, N. et al. Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424 (2011).
Powell, A. E. et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012).
Wong, V. W. et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nature Cell Biol. 14, 401–408 (2012).
Montgomery, R. K. et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl Acad. Sci. USA 108, 179–184 (2011).
Metcalfe, C., Kljavin, N. M., Ybarra, R. & de Sauvage, F. J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14, 149–159 (2014).
Yan, K. S. et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl Acad. Sci. USA 109, 466–471 (2012).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011). These authors show that Lgr5+ ISCs are dispensable for intestinal homeostasis and that loss of Lgr5+ cells stimulates expansion of Bmi1+ 'reserve' stem cells, which repopulate the crypt.
He, X. C. et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–ß-catenin signaling. Nature Genet. 36, 1117–1121 (2004).
Noah, T. K. & Shroyer, N. F. Notch in the intestine: regulation of homeostasis and pathogenesis. Annu. Rev. Physiol. 75, 263–288 (2013).
Buczacki, S. J. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013). These authors show that histone H2B label-retaining cells in the intestine are secretory progenitor cells that can revert to become stem cells following genotoxic injury.
van Es, J. H. et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature Cell Biol. 14, 1099–1104 (2012). This work shows that Dll1+ secretory progenitor cells can become activated by injury to repopulate the injured crypt.
Gerbe, F. et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 192, 767–780 (2011).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Durand, A. et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc. Natl Acad. Sci. USA 109, 8965–8970 (2012).
Kabiri, Z. et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215 (2014).
Nigro, G., Rossi, R., Commere, P. H., Jay, P. & Sansonetti, P. J. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15, 792–798 (2014).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Moore, S. R. et al. Robust circadian rhythms in organoid cultures from PERIOD2::LUCIFERASE mouse small intestine. Dis. Model. Mech. 7, 1123–1130 (2014).
Melendez, J. et al. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology 145, 808–819 (2013).
Van Landeghem, L. et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1111–G1132 (2012).
Middendorp, S. et al. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells 32, 1083–1091 (2014).
Fukuda, M. et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 28, 1752–1757 (2014).
Kozar, S. et al. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 13, 626–633 (2013).
Challen, G. A. et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nature Genet. 44, 23–31 (2012).
Shlush, L. I. et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506, 328–333 (2014).
Corces-Zimmerman, M. R., Hong, W. J., Weissman, I. L., Medeiros, B. C. & Majeti, R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc. Natl Acad. Sci. USA 111, 2548–2553 (2014).
Welch, J. S. et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 150, 264–278 (2012).
Krivtsov, A. V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature 442, 818–822 (2006).
Rosen, J. M. & Jordan, C. T. The increasing complexity of the cancer stem cell paradigm. Science 324, 1670–1673 (2009).
Kreso, A. & Dick, J. E. Evolution of the cancer stem cell model. Cell Stem Cell 14, 275–291 (2014).
Oguro, H., Ding, L. & Morrison, S. J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116 (2013).
Acknowledgements
Research in the Goodell laboratory is supported by the US National Institutes of Health (NIH) (grants DK092883 and CA183252), the Edward P. Evans Foundation, and the Samuel Waxman Cancer Research Foundation. Research in the Nguyen laboratory is supported by R01AR059122. Work in the Shroyer laboratory is supported by the US NIH grants DK092456, DK092306, CA1428260 and DK103117.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Quiescence
-
The state of being inactive. This usually implies limited mitotic activity and is sometimes referred to as dormancy.
- Niches
-
The local environments in which stem cells reside. Niches are thought to regulate stem cell activity through various types of interaction.
- CrePR
-
A Cre recombinase fused with a truncated progesterone receptor that translocates into the nucleus when the receptor binds to the progesterone antagonist RU486.
- CreER
-
A Cre recombinase fused with an oestrogen receptor that translocates into the nucleus when the receptor binds to the oestrogen antagonist tamoxifen. When it translocates to the nucleus, the Cre recombinase is activated and removes the sequences preceding the reporter gene, allowing expression of the reporter.
- Quiescent label-retaining cells
-
Cells that are labelled with a pulse of 5-bromo-2-deoxyuridine (BrdU) and that retain the BrdU label when followed for a certain amount of time.
Rights and permissions
About this article
Cite this article
Goodell, M., Nguyen, H. & Shroyer, N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat Rev Mol Cell Biol 16, 299–309 (2015). https://doi.org/10.1038/nrm3980
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3980
This article is cited by
-
A beneficial adaptive role for CHOP in driving cell fate selection during ER stress
EMBO Reports (2024)
-
A statistical method for quantifying progenitor cells reveals incipient cell fate commitments
Nature Methods (2024)
-
Isolation of Swine Bone Marrow Lin-/CD45-/CD133 + Cells and Cardio-protective Effects of its Exosomes
Stem Cell Reviews and Reports (2023)
-
Lin28a maintains a subset of adult muscle stem cells in an embryonic-like state
Cell Research (2023)
-
The universal stem cell
Leukemia (2022)