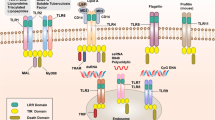
Key Points
-
Scavenger receptors constitute a heterogeneous family of receptors capable of recognizing danger-associated molecular patterns.
-
They are involved in the clearance of modified lipoproteins, such as the oxidized forms of low-density lipoprotein (LDL), which are key to the pathogenesis of atherosclerosis, and the β-amyloid fibrils associated with Alzheimer's disease.
-
Remarkably, scavenger receptors also recognize pathogen-associated molecular patterns and are therefore implicated in the innate immune response.
-
The ability to associate with a wide range of ligands is partly due to the structural diversity of the receptors that constitute the scavenger receptor family and also to their ability to associate with various co-receptors.
-
This article covers recent insights into the structural features of scavenger receptors, their modes of signalling and their functional roles in macrophage polarization and in the pathogenesis of various diseases.
Abstract
Scavenger receptors were originally identified by their ability to recognize and to remove modified lipoproteins; however, it is now appreciated that they carry out a striking range of functions, including pathogen clearance, lipid transport, the transport of cargo within the cell and even functioning as taste receptors. The large repertoire of ligands recognized by scavenger receptors and their broad range of functions are not only due to the wide range of receptors that constitute this family but also to their ability to partner with various co-receptors. The ability of individual scavenger receptors to associate with different co-receptors makes their responsiveness extremely versatile. This Review highlights recent insights into the structural features that determine the function of scavenger receptors and the emerging role that these receptors have in immune responses, notably in macrophage polarization and in the pathogenesis of diseases such as atherosclerosis and Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brown, M. S. & Goldstein, J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc. Natl Acad. Sci. USA 76, 3330–3337 (1979).
Brown, M. S., Goldstein, J. L., Krieger, M., Ho, Y. K. & Anderson, R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J. Cell Biol. 82, 597–613 (1979).
Greaves, D. R. & Gordon, S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 50, S282–S286 (2008).
Kzhyshkowska, J., Neyen, C. & Gordon, S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 217, 492–502 (2012).
Hansson, G. K. & Hermansson, A. The immune system in atherosclerosis. Nature Immunol. 12, 204–212 (2011).
Moore, Kathryn, J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Tabas, I., Williams, K. J. & Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116, 1832–1844 (2007).
Miller, Y. I. et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108, 235–248 (2011).
Hartvigsen, K. et al. The role of innate immunity in atherogenesis. J. Lipid Res. 50, S388–S393 (2008). In this study, the authors introduce the idea that oxidation-specific epitopes are DAMPs, which are major targets of many innate PRRs.
Shibata, M. et al. Type F scavenger receptor SREC-I interacts with advillin, a member of the gelsolin/villin family, and induces neurite-like outgrowth. J. Biol. Chem. 279, 40084–40090 (2004).
Kzhyshkowska, J., Gratchev, A. & Goerdt, S. Stabilin-1, a homeostatic scavenger receptor with multiple functions. J. Cell. Mol. Med. 10, 635–649 (2006).
Gu, B. J., Saunders, B. M., Petrou, S. & Wiley, J. S. P2X7 is a scavenger receptor for apoptotic cells in the absence of its ligand, extracellular ATP. J. Immunol. 187, 2365–2375 (2011).
Bonventre, J. V. & Yang, L. Kidney injury molecule-1. Curr. Opin. Crit. Care 16, 556–561 (2010).
Van Gorp, H., Delputte, P. L. & Nauwynck, H. J. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol. Immunol. 47, 1650–1660 (2010).
Areschoug, T., Gordon, S., Egesten, A., Schmidt, A. & Herwald, H. in Contributions to microbiology 45–60 (Karger, 2008).
Plüddemann, A., Mukhopadhyay, S. & Gordon, S. The interaction of macrophage receptors with bacterial ligands. Expert Rev. Mol. Med. 8, 1–25 (2006).
Plüddemann, A., Mukhopadhyay, S. & Gordon, S. Innate immunity to intracellular pathogens: macrophage receptors and responses to microbial entry. Immunol. Rev. 240, 11–24 (2011).
Krieger, M. The other side of scavenger receptors: pattern recognition for host defense. Curr. Opin. Lipidol. 8, 275–280 (1997).
Medzhitov, R. & Janeway, C. A. Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 (2002).
Mukhopadhyay, S., Plüddemann, A., Gordon, S. & Kishore, U. in Target pattern recognition in innate immunity 1–14 (Springer New York, 2009).
Areschoug, T. & Gordon, S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol. 11, 1160–1169 (2009).
Mukhopadhyay, S. & Gordon, S. The role of scavenger receptors in pathogen recognition and innate immunity. Immunobiology 209, 39–49 (2004).
Plüddemann, A., Neyen, C. & Gordon, S. Macrophage scavenger receptors and host-derived ligands. Methods 43, 207–217 (2007).
Suzuki, H. et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–296 (1997). This study shows that scavenger receptors — in particular SR-A1 — have an important role not only in host defence against pathogens but also in contributing to the generation of atherosclerotic lesions in vivo.
Taylor, P. R. et al. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23, 901–944 (2005).
Herrmann, M. et al. Clearance of fetuin-A−-containing calciprotein particles is mediated by scavenger receptor-A. Circ. Res. 111, 575–584 (2012).
Sun, M. et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem. 281, 4222–4230 (2006).
Palani, S. et al. Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages. Eur. J. Immunol. 41, 2052–2063 (2011).
Shimaoka, T. et al. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J. Leukoc. Biol. 75, 267–274 (2004).
Santiago-Garcia, J., Kodama, T. & Pitas, R. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J. Biol. Chem. 278, 6942–6946 (2003).
Murshid, A., Gong, J., Calderwood, S. K., Henderson, B. & Pockley, A. G. in Cellular trafficking of cell stress proteins in health and disease 215–227 (Springer Netherlands, 2012).
Feng, H. et al. Deficiency of scavenger receptor BI leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arterioscler. Thromb. Vasc. Biol. 31, 2543–2551 (2011).
Kzhyshkowska, J. Multifunctional receptor stabilin-1 in homeostasis and disease. Scientific World Journal 10, 2039–2053 (2010).
Park, L. et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 110, 3089–3094 (2013).
Podrez, E. A. et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature Med. 13, 1086–1095 (2007).
Silverstein, R. L. & Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2, re3 (2009).
Wiley, J. S., Sluyter, R., Gu, B. J., Stokes, L. & Fuller, S. J. The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78, 321–332 (2011).
Pal, S., Wu, L. & Kishore, U. Lessons from the fly: pattern recognition in Drosophila melanogaster. Target Pattern Recogn. Innate Immun. 653, 162–174 (2009).
Stuart, L. M. & Ezekowitz, R. A. Phagocytosis and comparative innate immunity: learning on the fly. Nature Rev. Immunol. 8, 131–141 (2008).
Cherry, S. & Silverman, N. Host-pathogen interactions in drosophila: new tricks from an old friend. Nature Immunol. 7, 911–917 (2006).
Song, L., Lee, C. & Schindler, C. Deletion of the murine scavenger receptor CD68. J. Lipid Res. 52, 1542–1550 (2011).
Fabriek, B. O. et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113, 887–892 (2009).
Murphy, J. E., Tedbury, P. R., Homer-Vanniasinkam, S., Walker, J. H. & Ponnambalam, S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 182, 1–15 (2005).
Sheikine, Y. & Sirsjö, A. CXCL16/SR-PSOX — A friend or a foe in atherosclerosis? Atherosclerosis 197, 487–495 (2008).
Sarrias, M.-R. et al. CD6 binds to pathogen-associated molecular patterns and protects from LPS-induced septic shock. Proc. Natl Acad. Sci. USA 104, 11724–11729 (2007).
Vera, J. et al. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc. Natl Acad. Sci. USA 106, 1506–1511 (2009).
Ichimura, T. et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 118, 1657–1668 (2008).
Gu, B. J. et al. P2X7 receptor-mediated scavenger activity of mononuclear phagocytes toward non-opsonized particles and apoptotic cells is inhibited by serum glycoproteins but remains active in cerebrospinal fluid. J. Biol. Chem. 287, 17318–17330 (2012).
Wiley, J. S. & Gu, B. J. A new role for the P2X7 receptor: a scavenger receptor for bacteria and apoptotic cells in the absence of serum and extracellular ATP. Purinerg. Signal 8, 579–586 (2012).
Ojala, J. R. M., Pikkarainen, T., Tuuttila, A., Sandalova, T. & Tryggvason, K. Crystal structure of the cysteine-rich domain of scavenger receptor, MARCO reveals the presence of a basic and an acidic cluster that both contribute to ligand recognition. J. Biol. Chem. 282, 16654–16666 (2007).
Rodamilans, B. et al. Crystal structure of the third extracellular domain of CD5 reveals the fold of a group B scavenger cysteine-rich receptor domain. J. Biol. Chem. 282, 12669–12677 (2007).
Ohki, I. et al. Crystal structure of human lectin-like, oxidized low-density lipoprotein receptor 1 ligand binding domain and its ligand recognition mode to OxLDL. Structure 13, 905–917 (2005). This structural analysis was the first to identify basic features of the ligand-recognition surface of a scavenger receptor (in this case, LOX1), which has an essential role in oxLDL binding.
Park, H., Adsit, F. G. & Boyington, J. C. The 1.4 angstrom crystal structure of the human oxidized low density lipoprotein receptor lox-1. J. Biol. Chem. 280, 13593–13599 (2005).
Feinberg, H., Taylor, M. E. & Weis, W. I. Scavenger receptor C-type lectin binds to the leukocyte cell surface glycan Lewisx by a novel mechanism. J. Biol. Chem. 282, 17250–17258 (2007).
Wilke, S., Krausze, J. & Büssow, K. Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: implications for the lysosomal glycocalyx. BMC Biol. 10, 62 (2012).
Kar, N. S., Ashraf, M. Z., Valiyaveettil, M. & Podrez, E. A. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor CD36. J. Biol. Chem. 283, 8765–8771 (2008).
Shimaoka, T. et al. Chemokines generally exhibit scavenger receptor activity through their receptor-binding domain. J. Biol. Chem. 279, 26807–26810 (2004).
Andersson, L. & Freeman, M. Functional changes in scavenger receptor binding conformation are induced by charge mutants spanning the entire collagen domain. J. Biol. Chem. 273, 19592–19601 (1998).
Kodama, T. et al. Collagenous macrophage scavenger receptors. Curr. Opin. Lipidol. 7, 287–291 (1996).
Podrez, E. A. et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 105, 1095–1108 (2000).
Boullier, A. et al. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J. Biol. Chem. 275, 9163–9169 (2000).
Nicholson, A. C., Frieda, S., Pearce, A. & Silverstein, R. L. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler. Thromb. Vasc. Biol. 15, 269–275 (1995).
Rigotti, A., Acton, S. L. & Krieger, M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 270, 16221–16224 (1995).
Ryeom, S. W., Silverstein, R. L., Scotto, A. & Sparrow, J. R. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J. Biol. Chem. 271, 20536–20539 (1996).
Podrez, E. A. et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 277, 38503–38516 (2002).
Podrez, E. A. et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277, 38517–38523 (2002).
Greenberg, M. E. et al. Oxidized phosphatidylserine- CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203, 2613–2625 (2006).
Gaidukov, L., Nager, A. R., Xu, S., Penman, M. & Krieger, M. Glycine dimerization motif in the N-terminal transmembrane domain of the high density lipoprotein receptor SR-BI required for normal receptor oligomerization and lipid transport. J. Biol. Chem. 286, 18452–18464 (2011).
Reaven, E., Cortez, Y., Leers-Sucheta, S., Nomoto, A. & Azhar, S. Dimerization of the scavenger receptor class B type I formation, function, and localization in diverse cells and tissues. J. Lipid Res. 45, 513–528 (2004).
Sankala, M. et al. Characterization of recombinant soluble macrophage scavenger receptor MARCO. J. Biol. Chem. 277, 33378–33385 (2002).
Rahaman, S. O. et al. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell. Metab. 4, 211–221 (2006).
Bull, H. A., Brickell, P. M. & Dowd, P. M. Src-related protein tyrosine kinases are physically associated with the surface antigen CD36 in human dermal microvascular endothelial cells. FEBS Lett. 351, 41–44 (1994).
Huang, M. M., Bolen, J. B., Barnwell, J. W., Shattil, S. J. & Brugge, J. S. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc. Natl Acad. Sci. USA 88, 7844–7848 (1991).
Jiménez, B. et al. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nature Med. 6, 41–48 (2000).
Shaw, A. S. et al. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol. Cell. Biol. 10, 1853–1862 (1990).
Turner, J. M. et al. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell 60, 755–765 (1990).
Moore, K. J. et al. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J. Biol. Chem. 277, 47373–47379 (2002). This is the initial report of a pro-inflammatory role of CD36 induced by β-amyloid.
Medeiros, L. A. et al. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J. Biol. Chem. 279, 10643–10648 (2004).
Stewart, C. R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature Immunol. 11, 155–161 (2010). This study establishes that the assembly of the TLR4–TLR6 heterodimer is regulated by CD36 and that signals from the CD36–TLR4–TLR6 complex characterize the mechanism by which atherogenic lipids and β-amyloid trigger sterile inflammation.
Calzada, M. J. et al. Identification of novel β1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J. Biol. Chem. 279, 41734–41743 (2004).
Chang, Y. & Finnemann, S. C. Tetraspanin CD81 is required for the αvβ5-integrin-dependent particle-binding step of RPE phagocytosis. J. Cell Sci. 120, 3053–3063 (2007).
Heit, B. et al. Multimolecular signaling complexes enable Syk-mediated signaling of CD36 internalization. Dev. Cell 24, 372–383 (2013).
Todt, J. C., Hu, B. & Curtis, J. L. The scavenger receptor SR-A I/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. J. Leukoc. Biol. 84, 510–518 (2008).
Yu, H. et al. Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-κB activation in macrophages. Biochim. Biophys. Acta 1823, 1192–1198 (2012).
Mukhopadhyay, S. et al. SR-A/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell surface limits TLR4 response to pathogens. Blood 117, 1319–1328 (2011).
Triantafilou, M. et al. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 281, 31002–31011 (2006).
Erdman, L. K. et al. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. J. Immunol. 183, 6452–6459 (2009).
Pupovac, A., Foster, C. M. & Sluyter, R. Human P2X7 receptor activation induces the rapid shedding of CXCL16. Biochem. Biophys. Res. Commun. 432, 626–631 (2013).
Gu, X. et al. The efficient cellular uptake of high density lipoprotein lipids via scavenger receptor class B type I requires not only receptor-mediated surface binding but also receptor-specific lipid transfer mediated by Its extracellular domain. J. Biol. Chem. 273, 26338–26348 (1998).
Rigotti, A., Miettinen, H. E. & Krieger, M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other Ttissues. Endocr. Rev. 24, 357–387 (2003).
Chroni, A., Nieland, T. J. F., Kypreos, K. E., Krieger, M. & Zannis, V. I. SR-BI mediates cholesterol efflux via its interactions with lipid-bound ApoE. Structural mutations in SR-BI diminish cholesterol efflux. Biochemistry 44, 13132–13143 (2005).
Nieland, T. J. F., Xu, S., Penman, M. & Krieger, M. Negatively cooperative binding of high-density lipoprotein to the HDL. receptor SR-BI. Biochemistry 50, 1818–1830 (2011).
Su, X. & Abumrad, N. A. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metabol. 20, 72–77 (2009).
Kennedy, D. J. & Kashyap, S. R. Pathogenic role of scavenger receptor CD36 in the metabolic syndrome and diabetes. Metab. Syndr. Relat. Disord. 9, 239–245 (2011).
Martin, C. et al. CD36 as a lipid sensor. Physiol. Behav. 105, 36–42 (2011).
Martin, C. et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE 6, e24014 (2011).
Pepino, M. Y., Love-Gregory, L., Klein, S. & Abumrad, N. A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 53, 561–566 (2012).
Helming, L., Winter, J. & Gordon, S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J. Cell Sci. 122, 453–459 (2009).
Anderson, J. M. Multinucleated giant cells. Curr. Opin. Hematol. 7, 40–47 (2000).
Byrd, T. F. Multinucleated giant cell formation induced by IFN-γ/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocyte monolayers. Cell. Immunol. 188, 89–96 (1998).
Reczek, D. et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of β-glucocerebrosidase. Cell 131, 770–783 (2007).
Blanz, J. et al. Disease-causing mutations within the lysosomal integral membrane protein type 2 (LIMP-2) reveal the nature of binding to its ligand β-glucocerebrosidase. Hum. Mol. Genet. 19, 563–572 (2010).
Desmond, M. J. et al. Tubular proteinuria in mice and humans lacking the intrinsic lysosomal protein SCARB2/Limp-2. Am. J. Physiol. Renal Physiol. 300, F1437–F1447 (2011).
Etzerodt, A. & Moestrup, S. K. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal. 18, 2352–2363 (2012).
Braun, M. et al. The CD6 scavenger receptor is differentially expressed on a CD56 natural killer cell subpopulation and contributes to natural killer-derived cytokine and chemokine secretion. J. Innate Immun. 3, 420–434 (2011).
Abel, S. et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-γ and TNF-α and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 172, 6362–6372 (2004).
Burdo, T. H. et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis. 204, 154–163 (2011).
Madsen, M. et al. Molecular characterization of the haptoglobin.hemoglobin receptor CD163. Ligand binding properties of the scavenger receptor cysteine-rich domain region. J. Biol. Chem. 279, 51561–51567 (2004).
Kristiansen, M. et al. Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 (2001). This reference identifies CD163 as the receptor for the haptoglobin–haemoglobin complex, which provides a mechanism for haemoglobin clearance.
Alonso, R. et al. Aberrant expression of CD6 on B-cell subsets from patients with Sjögren's syndrome. J. Autoimmun 35, 336–341 (2010).
Smith, E. R., Hanssen, E., McMahon, L. P. & Holt, S. G. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS ONE 8, e60904 (2013).
Chen, Y. et al. Defective microarchitecture of the spleen marginal zone and impaired response to a thymus-independent type 2 antigen in mice lacking scavenger receptors MARCO and SR-A. J. Immunol. 175, 8173–8180 (2005).
Karlsson, M. C. et al. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 198, 333–340 (2003).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nature Rev. Immunol. 8, 958–969 (2008).
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A. & Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185 (2013).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Oh, J. et al. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J. Biol. Chem. 287, 11629–11641 (2012).
Buechler, C. et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 67, 97–103 (2000).
Weaver, L. K., Pioli, P. A., Wardwell, K., Vogel, S. N. & Guyre, P. M. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J. Leukoc. Biol. 81, 663–671 (2007).
Xu, W. et al. Human peritoneal macrophages show functional characteristics of M-CSF-driven anti-inflammatory type 2 macrophages. Eur. J. Immunol. 37, 1594–1599 (2007).
Tippett, E. et al. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PloS ONE 6, e19968 (2011).
Ogden, C. A. et al. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt's lymphoma. J. Immunol. 174, 3015–3023 (2005).
Xu, W. et al. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood 107, 4930–4937 (2006).
Zizzo, G., Hilliard, B. A., Monestier, M. & Cohen, P. L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J. Immunol. 189, 3508–3520 (2012).
Fadok, V. A., Warner, M. L., Bratton, D. L. & Henson, P. M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αvβ3). J. Immunol. 161, 6250–6257 (1998).
Bover, L. C. et al. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J. Immunol. 178, 8183–8194 (2007).
Schaer, D. J., Alayash, A. I. & Buehler, P. W. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxid. Redox Signal. 9, 991–999 (2007).
Philippidis, P. et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circul. Res. 94, 119–126 (2004).
Józefowski, S. & Kobzik, L. Scavenger receptor A mediates H2O2 production and suppression of IL-12 release in murine macrophages. J. Leukoc.Biol. 76, 1066–1074 (2004). In this study the authors show that the scavenger receptors SR-A1 and MARCO are differentially regulated by M1-polarizing versus M2-polarizing factors. In addition, MARCO is shown to stimulate pro-inflammatory cytokine production, whereas SR-A1 has the opposite effect.
Józefowski, S., Arredouani, M., Sulahian, T. & Kobzik, L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J. Immunol. 175, 8032–8041 (2005). This study shows that the class B scavenger receptor CD36 is a selective sensor of microbial diacylated lipoproteins and LTA, which signal through the TLR2–TLR6 heterodimer.
Hoebe, K. et al. CD36 is a sensor of diacylglycerides. Nature 433, 523–527 (2005).
Kennedy, D. J. et al. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc. Res. 89, 604–613 (2011).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Mukhopadhyay, S. & Gordon, S. The role of scavenger receptors in pathogen recognition and innate immunity. Immunobiology 209, 39–49 (2004).
Peiser, L. et al. Identification of Neisseria meningitidis nonlipopolysaccharide ligands for class A macrophage scavenger receptor by using a novel assay. Infect. Immun. 74, 5191–5199 (2006).
Thomas, C. A. et al. Protection from lethal Gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J. Exp. Med. 191, 147–156 (2000).
Peiser, L. et al. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect. Immun. 70, 5346–5354 (2002).
Arredouani, M. S. et al. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J. Respir. Cell. Mol. Biol. 35, 474–478 (2006). This study shows that SR-A1 and MARCO recognize overlapping but distinct sets of endogenous and microbial ligands, which highlights the fine specificities of two related scavenger receptors.
Plüddemann, A. et al. SR-A, MARCO and TLRs differentially recognise selected surface proteins from Neisseria meningitidis: an example of fine specificity in microbial ligand recognition by innate immune receptors. J. Innate Immun. 1, 153–163 (2009).
Amiel, E. et al. Pivotal advance: Toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J. Leukoc. Biol. 85, 595–605 (2009).
Bowdish, D. M. E. et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 5, e1000474 (2009).
Dreux, M. et al. Receptor complementation and mutagenesis reveal SR-BI as an essential HCV entry factor and functionally imply its intra- and extra-cellular domains. PLoS Pathog. 5, e1000310 (2009).
Ploss, A. et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886 (2009).
Westhaus, S. et al. Characterization of the inhibition of hepatitis C virus entry by in vitro-generated and patient-derived oxidized low-density lipoprotein. Hepatology 57, 1716–1724 (2012).
Dao Thi, V. L. et al. Characterization of hepatitis C virus particle subpopulations reveals multiple usage of the scavenger receptor BI for entry steps. J. Biol. Chem. 287, 31242–31257 (2012).
Cox, J. V., Naher, N., Abdelrahman, Y. M. & Belland, R. J. Host HDL biogenesis machinery is recruited to the inclusion of Chlamydia trachomatis-infected cells and regulates chlamydial growth. Cell. Microbiol. 14, 1497–1512 (2012).
Yamayoshi, S. et al. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nature Med. 15, 798–801 (2009).
Yamayoshi, S. et al. Human SCARB2-dependent infection by coxsackievirus A7, A14, and A16 and enterovirus 71. J. Virol. 86, 5686–5696 (2012).
Yamayoshi, S., Ohka, S., Fujii, K. & Koike, S. Functional Comparison of SCARB2 and PSGL1 as Receptors for Enterovirus 71. J. Virol. 87, 3335–3347 (2013).
Stuart, L. M. et al. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J. Cell Biol. 170, 477–485 (2005).
Baranova, I. N. et al. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J. Immunol. 181, 7147–7156 (2008).
Patel, S. N. et al. Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo. J. Immunol. 178, 3954–3961 (2007).
Philips, J. A., Rubin, E. J. & Perrimon, N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science 309, 1251–1253 (2005).
Hawkes, M. et al. CD36 deficiency attenuates experimental mycobacterial infection. BMC Infect. Dis. 10, 299 (2010).
Santiago-Garcia, J., Mas-Oliva, J., Innerarity, T. & Pitas, R. Secreted forms of the amyloid-β precursor protein are ligands for the class A scavenger receptor. J. Biol. Chem. 276, 30655–30661 (2001).
Park, L. et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-β. Proc. Natl Acad. Sci. USA 108, 5063–5068 (2011).
Hansson, G. K. Inflammatory mechanisms in atherosclerosis. J. Thromb. Haemost. 7, 328–331 (2009).
Yang, Z. & Ming, X.-F. CD36: the common soil for inflammation in obesity and atherosclerosis? Cardiovasc. Res. 89, 485–486 (2011).
van Berkel, T. J. C. et al. Scavenger receptors: friend or foe in atherosclerosis? Curr. Opin. Lipidol. 16, 525–535 (2005).
Moore, K. J. & Freeman, M. W. Scavenger receptors in atherosclerosis beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 26, 1702–1711 (2006).
Gautam, S. & Banerjee, M. The macrophage Ox-LDL receptor, CD36 and its association with type II diabetes mellitus. Mol. Genet. Metab. 102, 389–398 (2011).
Nosadini, R. & Tonolo, G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 21, 79–85 (2011).
Wilkinson, K. & El Khoury, J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer's disease. Int. J. Alzheimers Dis. 2012, 1–10 (2012).
Yamanaka, M. et al. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 32, 17321–17331 (2012).
Mathieu, P., Pibarot, P. & Després, J. P. Metabolic syndrome: the danger signal in atherosclerosis. Vasc. Health Risk Manag. 2, 285–302 (2006).
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature Rev. Immunol. 10, 36–46 (2010).
Xu, S. et al. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell. Mol. Life Sci. 70, 2859–2872 (2013).
Silverstein, R. L., Li, W., Park, Y. M. & Rahaman, S. O. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans. Am. Clin. Climatol. Assoc. 121, 206–220 (2010).
Mitra, S., Goyal, T. & Mehta, J. L. Oxidized LDL, LOX-1 and Atherosclerosis. Cardiovasc. Drugs Ther. 25, 419–429 (2011).
Kunjathoor, V. V. et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277, 49982–49988 (2002). This study shows the pro-atherogenic role of CD36 in vivo.
Sun, B. et al. Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J. Lipid Res. 48, 2560–2570 (2007).
Kuchibhotla, S. et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 78, 185–196 (2008).
Stewart, C. R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature Immunol. 11, 155–161 (2010).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature Immunol. 14, 812–820 (2013).
Björkbacka, H. et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nature Med. 10, 416–421 (2004).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA 101, 10679–10684 (2004).
Nagy, L., Tontonoz, P., Alvarez, J. G., Chen, H. & Evans, R. M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 93, 229–240 (1998).
Bujold, K. et al. CD36-mediated cholesterol efflux is associated with PPARγ activation via a MAPK-dependent COX-2 pathway in macrophages. Cardiovasc. Res. 83, 457–464 (2009).
Tontonoz, P., Nagy, L., Alvarez, J. G., Thomazy, V. A. & Evans, R. M. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252 (1998).
Scull, C. M. & Tabas, I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 2792–2797 (2011).
Tandon, N. N., Lipsky, R. H., Burgess, W. H. & Jamieson, G. A. Isolation and characterization of platelet glycoprotein IV (CD36). J. Biol. Chem. 264, 7570–7575 (1989).
Podrez, E. A. et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature Med. 13, 1086–1095 (2007).
Rhainds, D. & Brissette, L. The role of scavenger receptor class B type I (SR-BI) in lipid trafficking: Defining the rules for lipid traders. Int. J. Biochem. Cell Biol. 36, 39–77 (2004).
Carvalho, A. C., Colman, R. W. & Lees, R. S. Platelet function in hyperlipoproteinemia. N. Engl. J. Med. 290, 434–438 (1974). This report establishes SR-B1 as a bona fide HDL receptor.
Stuart, M. J., Gerrard, J. M. & White, J. G. Effect of cholesterol on production of thromboxane b2 by platelets in vitro. N. Engl. J. Med. 302, 6–10 (1980).
Davì, G. et al. Increased levels of soluble P-selectin in hypercholesterolemic patients. Circulation 97, 953–957 (1998).
Davì, G. et al. Increased thromboxane biosynthesis in type IIa hypercholesterolemia. Circulation 85, 1792–1798 (1992).
Ghosh, A. et al. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J. Clin. Invest. 118, 1934–1943 (2008).
Chen, M. et al. Activation-dependent surface expression of LOX-1 in human platelets. Biochem. Biophys. Res. Commun. 282, 153–158 (2001).
Marwali, M. R. et al. Modulation of ADP-induced platelet activation by aspirin and pravastatin: role of lectin-like oxidized low-density lipoprotein receptor-1, nitric oxide, oxidative stress, and inside-out integrin signaling. J. Pharmacol. Exp. Ther. 322, 1324–1332 (2007).
Kozarsky, K. F., Donahee, M. H., Glick, J. M., Krieger, M. & Rader, D. J. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 20, 721–727 (2000).
Braun, A. et al. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 90, 270–276 (2002).
Zhang, W. et al. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 108, 2258–2263 (2003).
Mineo, C. & Shaul, P. W. Functions of scavenger receptor class B, type I in atherosclerosis. Curr. Opin. Lipidol 23, 487–493 (2012).
Imachi, H. et al. Expression of human scavenger receptor B1 on and in human platelets. Arterioscler. Thromb. Vasc. Biol. 23, 898–904 (2003).
Valiyaveettil, M. et al. Oxidized high-density lipoprotein inhibits platelet activation and aggregation via scavenger receptor BI. Blood 111, 1962–1971 (2008).
Acton, S. et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271, 518–520 (1996).
Rosenson, R. S. et al. Cholesterol efflux and atheroprotection advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919 (2012).
Chadwick, A. C. & Sahoo, D. Functional genomics of the human high-density lipoprotein receptor scavenger receptor BI: an old dog with new tricks. Curr. Opin. Endocrinol. Diabetes Obes. 20, 124–131 (2013).
Rhainds, D. et al. Localization and regulation of SR-BI in membrane rafts of HepG2 cells. J. Cell Sci. 117, 3095–3105 (2004).
Silver, D. L. SR-BI and protein–protein interactions in hepatic high density lipoprotein metabolism. Rev. Endocr. Metab. Disord. 5, 327–333 (2004).
Yancey, P. G. et al. Severely altered cholesterol homeostasis in macrophages lacking apoE and SR-BI. J. Lipid Res. 48, 1140–1149 (2007).
Manichaikul, A. et al. Association of SCARB1 variants with subclinical atherosclerosis and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 1991–1999 (2012).
Naj, A. C. et al. Association of scavenger receptor class B type I polymorphisms with subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Circ. Cardiovasc. Genet. 3, 47–52 (2010).
Aronow, W. S. Antiplatelet therapy in peripheral arterial disease. Curr. Drug Targets Cardiovasc. Haematol. Disord. 4, 265–267 (2004).
Aslanian, A. M. & Charo, I. F. Targeted disruption of the scavenger receptor and chemokine CXCL16 accelerates atherosclerosis. Circulation 114, 583–590 (2006).
Sakaguchi, H. et al. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest. 78, 423–434 (1998).
Holland, W. L. et al. Lipid mediators of insulin resistance. Nutr. Rev. 65, S39–46 (2007).
Holloway, G. P. et al. In obese rat muscle transport of palmitate is increased and is channeled to triacylglycerol storage despite an increase in mitochondrial palmitate oxidation. Am. J. Physiol. Endocrinol. Metab. 296, E738–E747 (2009).
Glatz, J. F., Luiken, J. J. & Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90, 367–417 (2010).
Bonen, A. et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 18, 1144–1146 (2004).
Coort, S. L. M. et al. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol. Cell. Biochem. 239, 213–219 (2002).
Pelsers, M. M. et al. Skeletal muscle fatty acid transporter protein expression in type 2 diabetes patients compared with overweight, sedentary men and age-matched, endurance-trained cyclists. Acta Physiol. (Oxf.) 190, 209–219 (2007).
Bokor, S. et al. Single-nucleotide polymorphism of CD36 locus and obesity in European adolescents. Obesity (Silver Spring) 18, 1398–1403 (2010).
Heni, M. et al. Variants in the CD36 gene locus determine whole-body adiposity, but have no independent effect on insulin sensitivity. Obesity (Silver Spring) 19, 1004–1009 (2011).
Love-Gregory, L. et al. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum. Mol. Genet. 17, 1695–1704 (2008).
Leprêtre, F., Cheyssac, C., Amouyel, P., Froguel, P. & Helbecque, N. A promoter polymorphism in CD36 is associated with an atherogenic lipid profile in a French general population. Atherosclerosis 173, 375–377 (2004).
Ma, X. et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum. Mol. Genet. 13, 2197–2205 (2004).
Leprêtre, F. et al. Genetic study of the CD36 gene in a French diabetic population. Diabetes Metab. 30, 459–463 (2004).
Corpeleijn, E. et al. Direct association of a promoter polymorphism in the CD36/FAT fatty acid transporter gene with Type 2 diabetes mellitus and insulin resistance. Diabet. Med. 23, 907–911 (2006).
Alkhatatbeh, M. J., Enjeti, A. K., Acharya, S., Thorne, R. F. & Lincz, L. F. The origin of circulating CD36 in type 2 diabetes. Nutr. Diabetes 3, e59 (2013).
Zhu, W., Li, W. & Silverstein, R. L. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood 119, 6136–6144 (2012).
Ghosh, A. et al. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood 117, 6355–6366 (2011).
Heneka, M. T. & O'Banion, M. K. Inflammatory processes in Alzheimer's disease. J. Neuroimmunol. 184, 69–91 (2007).
Chung, H., Brazil, M. I., Irizarry, M. C., Hyman, B. T. & Maxfield, F. R. Uptake of fibrillar β-amyloid by microglia isolated from MSR-A (type I and type II) knockout mice. Neuroreport 12, 1151–1154 (2001).
El Khoury, J. B. et al. CD36 mediates the innate host response to β-amyloid. J. Exp. Med. 197, 1657–1666 (2003).
Acknowledgements
We thank A. Darszon and W. Trimble for their helpful comments. The original work in the authors' laboratory is supported by the Heart and Stroke Foundation of Canada, Cystic Fibrosis Canada and the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
Supplementary information
Supplementary information S1 (table)
Summary of the tissue distribution, ligand specificity, suggested function and diseases associated with scavenger receptors. (PDF 264 kb)
Glossary
- Danger-associated molecular patterns
-
(DAMPs). Molecules that are released in association with tissue damage or injury; they promote inflammation and tissue repair by triggering pattern-recognition receptors. DAMPs can be released from the degraded stroma (for example, hyaluronan), from the cell nucleus (for example, high-mobility group box 1 protein) and from the cell cytosol (for example, ATP, uric acid, S100 molecules and heat-shock proteins).
- Pathogen-associated molecular patterns
-
(PAMPs). Conserved microbial structures that are recognized by innate receptors, including Toll-like receptors.
- Pattern recognition receptors
-
(PRRs). Host receptors (such as Toll-like receptors) that are able to sense pathogen-associated molecular patterns and to initiate signalling cascades (often involving the activation of nuclear factor-κB) that lead to an innate immune response.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A structural motif containing a tyrosine residue that is found in the cytoplasmic tails of several signalling molecules. The consensus sequence consists of Tyr–X–X–Leu or Tyr–X–X–Ile. The tyrosine is a target for phosphorylation by SRC tyrosine kinases and for the subsequent binding of proteins containing SRC homology 2 domains.
Rights and permissions
About this article
Cite this article
Canton, J., Neculai, D. & Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 13, 621–634 (2013). https://doi.org/10.1038/nri3515
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3515
This article is cited by
-
Functional role of Ash2l in oxLDL induced endothelial dysfunction and atherosclerosis
Cellular and Molecular Life Sciences (2024)
-
The application of MARCO for immune regulation and treatment
Molecular Biology Reports (2024)
-
Blood monocyte and dendritic cell profiles among people living with HIV with Mycobacterium tuberculosis co-infection
BMC Immunology (2023)
-
Effect of the gut microbiota and their metabolites on postoperative intestinal motility and its underlying mechanisms
Journal of Translational Medicine (2023)
-
Promoting AMPK/SR-A1-mediated clearance of HMGB1 attenuates chemotherapy-induced peripheral neuropathy
Cell Communication and Signaling (2023)