Key Points
-
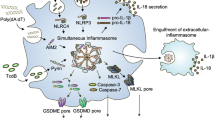
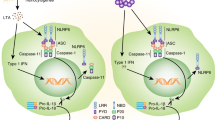
In addition to mediating the maturation and secretion of the cytokines interleukin-1β (IL-1β) and IL-18, caspase 1 activation by inflammasome complexes controls a set of non-canonical effectors that might contribute to the immune response during infection and autoimmunity. These mechanisms include unconventional protein secretion, pyroptosis, regulation of metabolic pathways and restriction of bacterial replication.
-
Caspase 1 activation in macrophages, epithelial cells and keratinocytes drives unconventional protein secretion of leaderless cytokines such as IL-1α, IL-1β and IL-18, growth factors such as fibroblast growth factor 2 and possibly damage-associated molecular patterns such as high mobility group box 1. After their release into the extracellular environment, these factors can enhance inflammatory and healing responses.
-
Infected myeloid cells can remove intracellular replication niches for pathogens by inducing pyroptosis, a specialized caspase 1-dependent cell death programme. Pyroptosis is accompanied by osmotic lysis and the release of the intracellular content into the extracellular milieu, and this is thought (together with other inflammasome functions) to render it an inherently pro-inflammatory cell death mode. Pyroptosis is thought to confer resistance to infection with intracellular pathogens in vivo, illustrating the importance of this cell death mode for host defence.
-
Caspase 1 can cleave poly(ADP-ribose) polymerase 1 (PARP1) and glycolysis enzymes (such as glyceraldehyde-3-phosphate dehydrogenase) to preserve ATP energy stores and to decrease the metabolic rate of infected cells. As such, caspase 1-mediated targeting of bioenergetic pathways might help to preserve cellular energy stores during infection.
-
Caspase 1 activates lipid metabolic pathways in fibroblasts intoxicated with pore-forming toxins or infected with bacteria that produce these toxins. This leads to the repair of toxin-induced damage to the plasma membrane and promotes cell survival.
-
In a process that proceeds independently of IL-1β and IL-18, caspase 1-mediated activation of caspase 7, an executioner caspase, contributes to the restriction of Legionella pneumophila replication in infected macrophages. In vivo studies have shown the importance of this inflammasome pathway for host defence against L. pneumophila infection in the lungs.
Abstract
Caspase 1 activation by inflammasome complexes in response to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) induces the maturation and secretion of the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18. Recent reports have begun to identify additional inflammasome effector mechanisms that proceed independently of IL-1β and IL-18. These include the induction of pyroptotic cell death, the restriction of bacterial replication, the activation of lipid metabolic pathways for cell repair and the secretion of DAMPs and leaderless cytokines. These non-canonical functions of caspase 1 illustrate the diverse mechanisms by which inflammasomes might contribute to innate immunity, repair responses and host defence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Villani, A. C. et al. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nature Genet. 41, 71–76 (2009).
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Allen, I. C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Bauer, C. et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59, 1192–1199 (2010).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Jin, Y. et al. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 356, 1216–1225 (2007).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Magitta, N. F. et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison's disease and type 1 diabetes. Genes Immun. 10, 120–124 (2009).
Larsen, C. M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nature Genet. 29, 301–305 (2001).
Lamkanfi, M. & Dixit, V. M. The inflammasomes. PLoS Pathog. 5, e1000510 (2009).
Kawai, T. & Akira, S. TLR signaling. Cell Death Differ. 13, 816–825 (2006).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nature Rev. Immunol. 10, 210–215 (2010).
Lamkanfi, M. & Dixit, V. M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 227, 95–105 (2009).
Gu, Y. et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275, 206–209 (1997).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267, 2000–2003 (1995).
Ghayur, T. et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386, 619–623 (1997).
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Joosten, L. A. et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1β. Arthritis Rheum. 60, 3651–3662 (2009).
Irmler, M. et al. Granzyme A is an interleukin 1β-converting enzyme. J. Exp. Med. 181, 1917–1922 (1995).
Maelfait, J. et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J. Exp. Med. 205, 1967–1973 (2008).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
Kanneganti, T. D. Central roles of NLRs and inflammasomes in viral infection. Nature Rev. Immunol. 10, 688–698 (2010).
Sims, J. E. & Smith, D. E. The IL-1 family: regulators of immunity. Nature Rev. Immunol. 10, 89–102 (2010).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Hoshino, T. et al. Cutting edge: IL-18-transgenic mice: in vivo evidence of a broad role for IL-18 in modulating immune function. J. Immunol. 166, 7014–7018 (2001).
Nakanishi, K., Yoshimoto, T., Tsutsui, H. & Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19, 423–474 (2001).
Weaver, C. T., Harrington, L. E., Mangan, P. R., Gavrieli, M. & Murphy, K. M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 (2006).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005).
Hoffman, H. M. et al. Efficacy and safety of rilonacept (interleukin-1 trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 58, 2443–2452 (2008).
Lachmann, H. J. et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360, 2416–2425 (2009).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010). This paper describes the role of the NLRP3 and NLRC4 inflammasomes in extracellular release of HMGB1.
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunol. 11, 1136–1142 (2010). This paper describes the crucial role of caspase 1-mediated cell death as a host defence mechanism against bacterial pathogens.
Henry, T. & Monack, D. M. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell. Microbiol. 9, 2543–2551 (2007). This paper describes the increased susceptibility of caspase 1-deficient mice to infection with Francisella tularensis compared with mice lacking IL-1β and IL-18.
Trombetta, E. S. & Parodi, A. J. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell. Dev. Biol. 19, 649–676 (2003).
Lee, M. C., Miller, E. A., Goldberg, J., Orci, L. & Schekman, R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell. Dev. Biol. 20, 87–123 (2004).
Nickel, W. & Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nature Rev. Mol. Cell Biol. 10, 148–155 (2009).
Rubartelli, A., Cozzolino, F., Talio, M. & Sitia, R. A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J. 9, 1503–1510 (1990).
Sutterwala, F. S. et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006).
Keller, M., Ruegg, A., Werner, S. & Beer, H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008). This paper characterizes the role of caspase 1 in the secretion of IL-1α and FGF2.
Becker, C. E., Creagh, E. M. & O'Neill, L. A. Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1β secretion. J. Biol. Chem. 284, 34531–34537 (2009).
Salvesen, G. S. & Riedl, S. J. Caspase mechanisms. Adv. Exp. Med. Biol. 615, 13–23 (2008).
Riedl, S. J. & Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nature Rev. Mol. Cell Biol. 5, 897–907 (2004).
Lamkanfi, M. & Dixit, V. M. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe 8, 44–54 (2010).
Strasser, A., O'Connor, L. & Dixit, V. M. Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000).
Taylor, R. C., Cullen, S. P. & Martin, S. J. Apoptosis: controlled demolition at the cellular level. Nature Rev. Mol. Cell Biol. 9, 231–241 (2008).
Kazama, H. et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29, 21–32 (2008).
Savill, J., Dransfield, I., Gregory, C. & Haslett, C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nature Rev. Immunol. 2, 965–975 (2002).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Lamkanfi, M. et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol. Cell. Proteomics 7, 2350–2363 (2008). This paper describes caspase 7 as a downstream effector of the NLRP3 and NLRC4 inflammasomes.
Fink, S. L. & Cookson, B. T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825 (2006).
Lamkanfi, M. et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Chen, Y., Smith, M. R., Thirumalai, K. & Zychlinsky, A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15, 3853–3860 (1996).
Hilbi, H. et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273, 32895–32900 (1998).
Monack, D. M., Detweiler, C. S. & Falkow, S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell. Microbiol. 3, 825–837 (2001). This paper reports that pyroptosis proceeds independently of IL-1β and IL-18.
van der Velden, A. W., Velasquez, M. & Starnbach, M. N. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J. Immunol. 171, 6742–6749 (2003).
Suzuki, T. et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 3, e111 (2007).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunol. 7, 569–575 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nature Immunol. 7, 576–582 (2006).
Amer, A. et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281, 35217–35223 (2006).
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3080 (2010).
Sutterwala, F. S. et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204, 3235–3245 (2007).
Boyden, E. D. & Dietrich, W. F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature Genet. 38, 240–244 (2006).
Terra, J. K. et al. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J. Immunol. 184, 17–20 (2010).
Munoz-Planillo, R., Franchi, L., Miller, L. S. & Nunez, G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 183, 3942–3948 (2009).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Sauer, J. D. et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7, 412–419 (2010).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature Immunol. 11, 385–393 (2010).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature Immunol. 11, 395–402 (2010).
Jones, J. W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl Acad. Sci. USA 107, 9771–9776 (2010).
Wu, J., Fernandes-Alnemri, T. & Alnemri, E. S. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J. Clin. Immunol. 30, 693–702 (2010).
Warren, S. E. et al. Cutting edge: cytosolic bacterial DNA activates the inflammasome via Aim2. J. Immunol. 185, 818–821 (2010).
Tsuchiya, K. et al. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J. Immunol. 185, 1186–1195 (2010).
Mariathasan, S., Weiss, D. S., Dixit, V. M. & Monack, D. M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049 (2005).
Monack, D. M., Raupach, B., Hromockyj, A. E. & Falkow, S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl Acad. Sci. USA 93, 9833–9838 (1996).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Malireddi, R. K., Ippagunta, S., Lamkanfi, M. & Kanneganti, T. D. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J. Immunol. 185, 3127–3130 (2010). This paper describes the inflammasome-dependent cleavage of PARP1 during pyroptosis.
Akhter, A. et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5, e1000361 (2009). This paper describes the role of caspase 7 activation by the NLRC4 inflammasome in restricting Legionella replication.
Lakhani, S. A. et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851 (2006).
Shao, W., Yeretssian, G., Doiron, K., Hussain, S. N. & Saleh, M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282, 36321–36329 (2007). This paper describes glycolysis enzymes as substrates of caspase 1.
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Gurcel, L., Abrami, L., Girardin, S., Tschopp, J. & van der Goot, F. G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145 (2006). This paper reports that inflammasomes activate lipid metabolic pathways.
Gonzalez, M. R., Bischofberger, M., Pernot, L., van der Goot, F. G. & Freche, B. Bacterial pore-forming toxins: the (w)hole story? Cell. Mol. Life Sci. 65, 493–507 (2008).
McCoy, A. J. et al. Cytotoxins of the human pathogen Aeromonas hydrophila trigger, via the NLRP3 inflammasome, caspase-1 activation in macrophages. Eur. J. Immunol. 40, 2797–2803 (2010).
Lamkanfi, M. et al. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J. Immunol. 178, 8022–8027 (2007).
Zamboni, D. S. et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nature Immunol. 7, 318–325 (2006).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Fischer, U., Janicke, R. U. & Schulze-Osthoff, K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 10, 76–100 (2003).
Lamkanfi, M., Festjens, N., Declercq, W., Vanden Berghe, T. & Vandenabeele, P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 14, 44–55 (2007).
Boatright, K. M. et al. A unified model for apical caspase activation. Mol. Cell 11, 529–541 (2003).
Martinon, F. & Tschopp, J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 14, 10–22 (2007).
Pizzirani, C. et al. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood 109, 3856–3864 (2007).
Acknowledgements
This work was supported by European Union Framework Program 7 (Marie Curie grant 256432) and by the Fund for Scientific Research – Flanders.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Cryopyrinopathies
-
A spectrum of hereditary autoinflammatory diseases that are caused by mutations in the gene encoding NLR family, pyrin domain-containing 3 (NLRP3) that trigger continuous activation of the NLRP3 inflammasome. Based on the severity and spectrum of the symptoms — which can include urticarial skin rashes, prolonged episodes of fever, sensorineural hearing loss, headaches, cognitive deficits and renal amyloidosis — these diseases are classified as familial cold autoinflammatory syndrome, Muckle–Wells syndrome or chronic infantile neurological cutaneous articular syndrome.
- Proximity-induced autoactivation
-
A process in which two or more initiator caspases are brought sufficiently close to induce their autocatalytic activation. This process is thought to occur in large cytosolic protein complexes to which caspase zymogens are recruited by means of homotypic interactions between the caspase recruitment domain (CARD) or death effector domain (DED) motifs in their pro-domains and several bipartite adaptor molecules.
- NOD-like receptor
-
(NLR). The human NLR family comprises 22 members. They share a domain organization that usually includes an amino-terminal caspase recruitment domain (CARD) or pyrin domain (PYD), followed by an intermediary nucleotide-binding oligomerization domain (NOD) and carboxy-terminal leucine-rich repeat motifs. NLRs are thought to survey the host cytosol and intracellular compartments for pathogen- and damage-associated molecular patterns to activate signalling pathways that contribute to the host innate immune response.
- Pathogen-associated molecular pattern
-
(PAMP). A conserved pathogen molecule that is usually essential for microbial survival, and that contains either nucleic acid structures that are unique to microorganisms or cell wall components (such as lipopolysaccharide and flagellin) that are not found in mammalian cells. PAMPs are ligands for receptors of the host innate immune system.
- Damage-associated molecular pattern
-
(DAMP). A molecule that is produced or released from host cells upon cellular stress, damage or non-physiological cell death. DAMPs are also referred to as 'alarmins' and are thought to be responsible for the initiation and perpetuation of inflammatory responses and tissue repair under non-infectious (sterile) conditions. Examples include high-mobility group box 1 (HMGB1), ATP, uric acid and heat-shock proteins.
- Unconventional protein secretion
-
The secretion of cytoplasmic and nuclear proteins into the extracellular space through an incompletely understood mechanism that does not require the translocation apparatus of the classical endoplasmic reticulum (ER)–Golgi secretion pathway. Proteins that are secreted through this route include interleukin-1α (IL-1α), IL-1β, IL-18, fibroblast growth factor 2, galectin 1, galectin 3 and possibly high-mobility group box 1.
- Pyroptosis
-
A specialized form of programmed cell death that requires caspase 1 activity. It is characterized by cytoplasmic swelling, early plasma membrane rupture, nuclear condensation and internucleosomal DNA fragmentation. The cytoplasmic content is released into the extracellular space, and this is thought to augment inflammatory and repair responses. Pyroptosis occurs in myeloid cells infected with pathogenic bacteria, and it might affect cells of the central nervous system and the cardiovascular system under ischaemic conditions.
- Type III and type IV secretion
-
Two of at least six specialized secretion systems by which Gram-negative pathogens can deliver virulence factors into eukaryotic host cells. Pathogenic bacteria such as Shigella, Salmonella, Yersinia, Chlamydia and Pseudomonas spp. all make use of a type III secretion system to infect host cells and to modulate signalling pathways. By contrast, pathogens such as Helicobacter pylori, Legionella pneumophila and Bordetella pertussis make use of a type IV secretion system for the horizontal transfer of plasmid DNA containing antibiotic resistance genes and to inject effector proteins into eukaryotic host cells.
- Glycolysis
-
A metabolic pathway that generates the cellular high-energy store ATP by oxidizing glucose to pyruvate. In eukaryotic cells, pyruvate is further oxidized into CO2 and H2O in a process known as 'aerobic respiration'. This results in a net yield of 36–38 molecules of ATP per metabolized molecule of glucose.
- Autophagosome
-
A double-membrane-bound vesicle that is used by eukaryotic cells to target protein aggregates, damaged organelles and invading microorganisms for digestion by lysosomal hydrolases. This catabolic process allows recycling of cellular components and is thought to contribute to cell death, cell survival during starvation, cellular differentiation and host defence against infectious agents.
Rights and permissions
About this article
Cite this article
Lamkanfi, M. Emerging inflammasome effector mechanisms. Nat Rev Immunol 11, 213–220 (2011). https://doi.org/10.1038/nri2936
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2936
This article is cited by
-
Regulation of NLRP3 Inflammasome Activation and Inflammatory Exosome Release in Podocytes by Acid Sphingomyelinase During Obesity
Inflammation (2023)
-
Effects of therapeutic plasma exchange on the endothelial glycocalyx in septic shock
Intensive Care Medicine Experimental (2021)
-
Gasdermin E-derived caspase-3 inhibitors effectively protect mice from acute hepatic failure
Acta Pharmacologica Sinica (2021)
-
Pyroptosis: mechanisms and diseases
Signal Transduction and Targeted Therapy (2021)
-
Schisandrin B Attenuates Airway Inflammation and Airway Remodeling in Asthma by Inhibiting NLRP3 Inflammasome Activation and Reducing Pyroptosis
Inflammation (2021)