Key Points
-
The extracellular matrix (ECM) is an ideal therapeutic substrate for functional remodelling of damaged gastrointestinal (GI) tissue, owing to its composition and roles in tissue development, wound repair and homeostasis
-
Components of the host response have been associated with ECM-induced site-appropriate constructive and functional tissue remodelling, including angiogenesis, innervation, stem cell recruitment, antimicrobial activity and modulation of the innate immune response
-
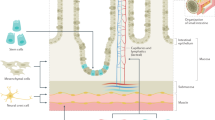
The GI tract presents distinct challenges for regenerative medicine, such as its requirement for motility and nutrient absorption, its diverse pH conditions and its tissue-specific 3D ultrastructure
-
ECM bioscaffolds comprise structural and functional molecules that are secreted by resident cells, and ECM bioscaffolds have been prepared from the decellularization of tissues from the oesophagus, small intestine, stomach and colon
-
Despite the challenges imposed by the GI tract, the use of ECM bioscaffolds for the reconstruction and regeneration of GI tissues has shown promise in preclinical and early clinical studies
-
Several studies have highlighted the potential benefits of using tissue-specific ECM bioscaffolds to facilitate the constructive and functional regeneration of tissues of the GI tract
Abstract
The synthesis and secretion of components that constitute the extracellular matrix (ECM) by resident cell types occur at the earliest stages of embryonic development, and continue throughout life in both healthy and diseased physiological states. The ECM consists of a complex mixture of insoluble and soluble functional components that are arranged in a tissue-specific 3D ultrastructure, and it regulates numerous biological processes, including angiogenesis, innervation and stem cell differentiation. Owing to its composition and influence on embryonic development, as well as cellular and organ homeostasis, the ECM is an ideal therapeutic substrate for the repair of damaged or diseased tissues. Biologic scaffold materials that are composed of ECM have been used in various surgical and tissue-engineering applications. The gastrointestinal (GI) tract presents distinct challenges, such as diverse pH conditions and the requirement for motility and nutrient absorption. Despite these challenges, the use of homologous and heterologous ECM bioscaffolds for the focal or segmental reconstruction and regeneration of GI tissue has shown promise in early preclinical and clinical studies. This Review discusses the importance of tissue-specific ECM bioscaffolds and highlights the major advances that have been made in regenerative medicine strategies for the reconstruction of functional GI tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mecham, R. P. in Current Protocols in Cell Biology (eds Bonifacino, J.S. et al.) 10.1.1–10.1.16 (John Wiley & Sons, 2012).
Mouw, J. K., Ou, G. & Weaver, V. M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15, 771–785 (2014).
Arnold, M., Soerjomataram, I., Ferlay, J. & Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64, 381–387 (2015).
Midwood, K. S., Williams, L. V. & Schwarzbauer, J. E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 36, 1031–1037 (2004).
Nelson, C. M. & Bissell, M. J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287–309 (2006).
Calve, S., Odelberg, S. J. & Simon, H. G. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol. 344, 259–271 (2010).
Humphrey, J. D., Dufresne, E. R. & Schwartz, M. A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014).
Charras, G. & Sahai, E. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 15, 813–824 (2014).
Sun, Z., Guo, S. S. & Fassler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 215, 445–456 (2016).
Rosso, F., Giordano, A., Barbarisi, M. & Barbarisi, A. From cell–ECM interactions to tissue engineering. J. Cell. Physiol. 199, 174–180 (2004).
Larsen, M., Artym, V. V., Green, J. A. & Yamada, K. M. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463–471 (2006).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Harburger, D. S. & Calderwood, D. A. Integrin signalling at a glance. J. Cell Sci. 122, 159–163 (2009).
Alam, N. et al. The integrin-growth factor receptor duet. J. Cell. Physiol. 213, 649–653 (2007).
Kim, S. H., Turnbull, J. & Guimond, S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 209, 139–151 (2011).
Lussier, C., Basora, N., Bouatrouss, Y. & Beaulieu, J. F. Integrins as mediators of epithelial cell–matrix interactions in the human small intestinal mucosa. Microsc. Res. Tech. 51, 169–178 (2000).
Beaulieu, J. F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 31, 1–78 (1997).
Beaulieu, J. F. Integrins and human intestinal cell functions. Front. Biosci. 4, D310–D321 (1999).
Sicari, B. M. et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33, 5524–5533 (2012).
Tottey, S. et al. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 32, 128–136 (2011).
Butcher, D. T., Alliston, T. & Weaver, V. M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009).
Linskens, M. H. et al. Cataloging altered gene expression in young and senescent cells using enhanced differential display. Nucleic Acids Res. 23, 3244–3251 (1995).
Bissell, M. J., Hall, H. G. & Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 99, 31–68 (1982).
Xu, R., Boudreau, A. & Bissell, M. J. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 28, 167–176 (2009).
Gilkes, D. M., Bajpai, S., Chaturvedi, P., Wirtz, D. & Semenza, G. L. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829 (2013).
Kufaishi, H., Alarab, M., Drutz, H., Lye, S. & Shynlova, O. Static mechanical loading influences the expression of extracellular matrix and cell adhesion proteins in vaginal cells derived from premenopausal women with severe pelvic organ prolapse. Reprod. Sci. 23, 978–992 (2016).
Dziki, J. L. et al. The effect of mechanical loading upon extracellular matrix bioscaffold-mediated skeletal muscle remodeling. Tissue Eng. Part A http://dx.doi.org/10.1089/ten.TEA.2017.0011 (2017).
Lu, P., Takai, K., Weaver, V. M. & Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3, a005058 (2011).
Almalki, S. G. & Agrawal, D. K. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res. Ther. 7, 129 (2016).
Kaestner, K. Development, Differentiation, and Disease of the Luminal Gastrointestinal Tract Vol. 96 (Academic Press, 2010).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Simon-Assmann, P., Kedinger, M., De Arcangelis, A., Rousseau, V. & Simo, P. Extracellular matrix components in intestinal development. Experientia 51, 883–900 (1995).
Goke, M. & Podolsky, D. K. Regulation of the mucosal epithelial barrier. Baillieres Clin. Gastroenterol. 10, 393–405 (1996).
Goke, M., Zuk, A. & Podolsky, D. K. Regulation and function of extracellular matrix intestinal epithelial restitution in vitro. Am. J. Physiol. 271, G729–G740 (1996).
Shimshoni, E., Yablecovitch, D., Baram, L., Dotan, I. & Sagi, I. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 64, 367–372 (2015).
Speca, S., Giusti, I., Rieder, F. & Latella, G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 18, 3635–3661 (2012).
Spenle, C. et al. Dysregulation of laminins in intestinal inflammation. Pathol. Biol. (Paris) 60, 41–47 (2012).
Teller, I. C. & Beaulieu, J. F. Interactions between laminin and epithelial cells in intestinal health and disease. Expert Rev. Mol. Med. 3, 1–18 (2001).
Worthley, D. L., Giraud, A. S. & Wang, T. C. The extracellular matrix in digestive cancer. Cancer Microenviron. 3, 177–185 (2010).
Verma, S., Kesh, K., Ganguly, N., Jana, S. & Swarnakar, S. Matrix metalloproteinases and gastrointestinal cancers: impacts of dietary antioxidants. World J. Biol. Chem. 5, 355–376 (2014).
Yamaguchi, H. et al. Stromal fibroblasts mediate extracellular matrix remodeling and invasion of scirrhous gastric carcinoma cells. PLoS ONE 9, e85485 (2014).
Okamoto, R. & Watanabe, M. Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J. Gastroenterol. 39, 1–6 (2004).
Voytik-Harbin, S. L., Brightman, A. O., Kraine, M. R., Waisner, B. & Badylak, S. F. Identification of extractable growth factors from small intestinal submucosa. J. Cell. Biochem. 67, 478–491 (1997).
Fogar, P. et al. Neural cell adhesion molecule (N-CAM) in gastrointestinal neoplasias. Anticancer Res. 17, 1227–1230 (1997).
Herszenyi, L. et al. Serum cathepsin B and plasma urokinase-type plasminogen activator levels in gastrointestinal tract cancers. Eur. J. Cancer Prev. 17, 438–445 (2008).
Zhang, H. Y. et al. Expression of adhesion molecules and mucins in human and rhesus macaque gastrointestinal epithelial cells. Histol. Histopathol. 26, 1405–1413 (2011).
Jarvelainen, H., Sainio, A., Koulu, M., Wight, T. N. & Penttinen, R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol. Rev. 61, 198–223 (2009).
Stumpf, M. et al. Collagen distribution and expression of matrix metalloproteinases 1 and 13 in patients with anastomotic leakage after large-bowel surgery. Langenbecks Arch. Surg. 386, 502–506 (2002).
Stumpf, M. et al. Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery. Surgery 137, 229–234 (2005).
Angotti, R. et al. Gastric transposition as a valid surgical option for esophageal replacement in pediatric patients: experience from three Italian medical centers. Gastroenterol. Rep. (Oxf.) 5, 47–51 (2016).
Rieder, F., Brenmoehl, J., Leeb, S., Scholmerich, J. & Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 56, 130–139 (2007).
Mammen, J. M. & Matthews, J. B. Mucosal repair in the gastrointestinal tract. Crit. Care Med. 31, S532–S537 (2003).
Chibishev, A., Simonovska, N. & Shikole, A. Post-corrosive injuries of upper gastrointestinal tract. Prilozi 31, 297–316 (2010).
Olczyk, P., Mencner, L. & Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. Biomed. Res. Int. 2014, 747584 (2014).
Cai, J. & Terasaki, P. I. Humoral theory of transplantation: mechanism, prevention, and treatment. Hum. Immunol. 66, 334–342 (2005).
Terasaki, P. I. A personal perspective: 100-year history of the humoral theory of transplantation. Transplantation 93, 751–756 (2012).
Chinen, J. & Buckley, R. H. Transplantation immunology: solid organ and bone marrow. J. Allergy Clin. Immunol. 125, S324–S335 (2010).
Gilbert, T. W., Sellaro, T. L. & Badylak, S. F. Decellularization of tissues and organs. Biomaterials 27, 3675–3683 (2006).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Keane, T. J., Londono, R., Turner, N. J. & Badylak, S. F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33, 1771–1781 (2012).
Brown, N. H. Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb. Perspect. Biol. 3, a005082 (2011).
Exposito, J. Y., D'Alessio, M., Solursh, M. & Ramirez, F. Sea urchin collagen evolutionarily homologous to vertebrate pro-alpha 2(I) collagen. J. Biol. Chem. 267, 15559–15562 (1992).
Bernard, M. P. et al. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry 22, 5213–5223 (1983).
van der Rest, M. & Garrone, R. Collagen family of proteins. FASEB J. 5, 2814–2823 (1991).
Allman, A. J. et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation 71, 1631–1640 (2001).
Alicuben, E. T. & DeMeester, S. R. Onlay ventral hernia repairs using porcine non-cross-linked dermal biologic mesh. Hernia 18, 705–712 (2014).
Mase, V. J. Jr et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511 (2010).
Bejjani, G. K., Zabramski, J. & Durasis Study, G. Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review. J. Neurosurg. 106, 1028–1033 (2007).
Longo, U. G., Lamberti, A., Petrillo, S., Maffulli, N. & Denaro, V. Scaffolds in tendon tissue engineering. Stem Cells Int. 2012, 517165 (2012).
Salzberg, C. A. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann. Plast. Surg. 57, 1–5 (2006).
Badylak, S. F. et al. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng. Part A 17, 1643–1650 (2011).
O'Connor, L. et al. Efficacy of anal fistula plug in closure of Crohn's anorectal fistulas. Dis. Colon Rectum 49, 1569–1573 (2006).
Swinehart, I. T. & Badylak, S. F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. 245, 351–360 (2016).
Badylak, S. F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann. Biomed. Eng. 42, 1517–1527 (2014).
Londono, R. & Badylak, S. F. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann. Biomed. Eng. 43, 577–592 (2015).
Turner, N. J., Badylak, J. S., Weber, D. J. & Badylak, S. F. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J. Surg. Res. 176, 490–502 (2012).
Sicari, B. M. et al. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng. Part A 18, 1941–1948 (2012).
Agrawal, V. et al. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc. Natl Acad. Sci. USA 107, 3351–3355 (2010).
Beattie, A. J., Gilbert, T. W., Guyot, J. P., Yates, A. J. & Badylak, S. F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng. Part A 15, 1119–1125 (2009).
Sarikaya, A. et al. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 8, 63–71 (2002).
Brown, B. N., Ratner, B. D., Goodman, S. B., Amar, S. & Badylak, S. F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33, 3792–3802 (2012).
Daly, K. A. et al. Damage associated molecular patterns within xenogeneic biologic scaffolds and their effects on host remodeling. Biomaterials 33, 91–101 (2012).
Badylak, S. F., Freytes, D. O. & Gilbert, T. W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 5, 1–13 (2009).
Dearth, C. L. et al. The effect of terminal sterilization on the material properties and in vivo remodeling of a porcine dermal biologic scaffold. Acta Biomater. 33, 78–87 (2016).
Keane, T. J., Swinehart, I. T. & Badylak, S. F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84, 25–34 (2015).
Badylak, S. F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 12, 367–377 (2004).
Nagata, S., Hanayama, R. & Kawane, K. Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010).
Zheng, M. H. et al. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J. Biomed. Mater. Res. B Appl. Biomater. 73, 61–67 (2005).
Gilbert, T. W., Freund, J. M. & Badylak, S. F. Quantification of DNA in biologic scaffold materials. J. Surg. Res. 152, 135–139 (2009).
Wong, M. L. & Griffiths, L. G. Immunogenicity in xenogeneic scaffold generation: antigen removal versus decellularization. Acta Biomater. 10, 1806–1816 (2014).
Wong, M. L., Wong, J. L., Vapniarsky, N. & Griffiths, L. G. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials 92, 1–12 (2016).
Cissell, D. D., Hu, J. C., Griffiths, L. G. & Athanasiou, K. A. Antigen removal for the production of biomechanically functional, xenogeneic tissue grafts. J. Biomech. 47, 1987–1996 (2014).
Matuska, A. M. & McFetridge, P. S. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold; implications for stem cell adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 103, 397–406 (2015).
Keane, T. J. & Badylak, S. F. The host response to allogeneic and xenogeneic biological scaffold materials. J. Tissue Eng. Regen. Med. 9, 504–511 (2015).
Freytes, D. O., Tullius, R. S. & Badylak, S. F. Effect of storage upon material properties of lyophilized porcine extracellular matrix derived from the urinary bladder. J. Biomed. Mater. Res. B Appl. Biomater. 78, 327–333 (2006).
Freytes, D. O., Tullius, R. S., Valentin, J. E., Stewart-Akers, A. M. & Badylak, S. F. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J. Biomed. Mater. Res. A 87, 862–872 (2008).
Saldin, L. T., Cramer, M. C., Velankar, S. S., White, L. J. & Badylak, S. F. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 49, 1–15 (2016).
Freytes, D. O., Martin, J., Velankar, S. S., Lee, A. S. & Badylak, S. F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 29, 1630–1637 (2008).
Wolf, M. T. et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 33, 7028–7038 (2012).
Saxena, A. K., Ainoedhofer, H. & Hollwarth, M. E. Esophagus tissue engineering: in vitro generation of esophageal epithelial cell sheets and viability on scaffold. J. Pediatr. Surg. 44, 896–901 (2009).
Saxena, A. K., Kofler, K., Ainodhofer, H. & Hollwarth, M. E. Esophagus tissue engineering: hybrid approach with esophageal epithelium and unidirectional smooth muscle tissue component generation in vitro. J. Gastrointest. Surg. 13, 1037–1043 (2009).
Lee, M., Wu, B. M., Stelzner, M., Reichardt, H. M. & Dunn, J. C. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng. Part A 14, 1395–1402 (2008).
Araki, M. et al. Development of a new tissue-engineered sheet for reconstruction of the stomach. Artif. Organs 33, 818–826 (2009).
Nakase, Y. et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 12, 403–412 (2006).
Somara, S., Gilmont, R. R., Dennis, R. G. & Bitar, K. N. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 137, 53–61 (2009).
Zakhem, E., Raghavan, S., Gilmont, R. R. & Bitar, K. N. Chitosan-based scaffolds for the support of smooth muscle constructs in intestinal tissue engineering. Biomaterials 33, 4810–4817 (2012).
Kim, I. Y. et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 26, 1–21 (2008).
Stevens, M. M. Biomaterials for bone tissue engineering. Mater. Today 11, 18–25 (2008).
O'Brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater. Today 14, 88–95 (2011).
Alaribe, F. N., Manoto, S. L. & Motaung, S. C. K. M. Scaffolds from biomaterials: advantages and limitations in bone and tissue engineering. Biologia 71, 353–366 (2016).
Sarkar, K., Xue, Y. & Sant, S. in The Immune Response to Implanted Materials and Devices (ed. Corradetti, B.) 81–105 (Springer, 2017).
Zhu, Y., Leong, M. F., Ong, W. F., Chan-Park, M. B. & Chian, K. S. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 28, 861–868 (2007).
Bitar, K. N. & Raghavan, S. Intestinal tissue engineering: current concepts and future vision of regenerative medicine in the gut. Neurogastroenterol. Motil. 24, 7–19 (2012).
Bitar, K. N., Raghavan, S. & Zakhem, E. Tissue engineering in the gut: developments in neuromusculature. Gastroenterology 146, 1614–1624 (2014).
Speer, A. L., Sala, F. G., Matthews, J. A. & Grikscheit, T. C. Murine tissue-engineered stomach demonstrates epithelial differentiation. J. Surg. Res. 171, 6–14 (2011).
Levin, D. E. et al. Human tissue-engineered small intestine forms from postnatal progenitor cells. J. Pediatr. Surg. 48, 129–137 (2013).
Bitar, K. N. & Zakhem, E. Tissue engineering and regenerative medicine as applied to the gastrointestinal tract. Curr. Opin. Biotechnol. 24, 909–915 (2013).
Zisch, A. H., Lutolf, M. P. & Hubbell, J. A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 12, 295–310 (2003).
Lovett, M., Lee, K., Edwards, A. & Kaplan, D. L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15, 353–370 (2009).
Wenger, A. et al. Development and characterization of a spheroidal coculture model of endothelial cells and fibroblasts for improving angiogenesis in tissue engineering. Cells Tissues Organs 181, 80–88 (2005).
Finkenzeller, G., Torio-Padron, N., Momeni, A., Mehlhorn, A. T. & Stark, G. B. In vitro angiogenesis properties of endothelial progenitor cells: a promising tool for vascularization of ex vivo engineered tissues. Tissue Eng. 13, 1413–1420 (2007).
Rouwkema, J., Rivron, N. C. & van Blitterswijk, C. A. Vascularization in tissue engineering. Trends Biotechnol. 26, 434–441 (2008).
Vunjak-Novakovic, G. & Radisic, M. Cell seeding of polymer scaffolds. Methods Mol. Biol. 238, 131–146 (2004).
Dziki, J. L., Sicari, B. M., Wolf, M. T., Cramer, M. C. & Badylak, S. F. Immunomodulation and mobilization of progenitor cells by extracellular matrix bioscaffolds for volumetric muscle loss treatment. Tissue Eng. Part A 22, 1129–1139 (2016).
Agrawal, V. et al. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng. Part A 17, 2435–2443 (2011).
Crapo, P. M., Tottey, S., Slivka, P. F. & Badylak, S. F. Effects of biologic scaffolds on human stem cells and implications for CNS tissue engineering. Tissue Eng. Part A 20, 313–323 (2014).
Galvez-Monton, C. et al. Neoinnervation and neovascularization of acellular pericardial-derived scaffolds in myocardial infarcts. Stem Cell Res. Ther. 6, 108 (2015).
Zhang, X. et al. Functional neovascularization in tissue engineering with porcine acellular dermal matrix and human umbilical vein endothelial cells. Tissue Eng. Part C Methods 17, 423–433 (2011).
Dziki, J. et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. NPJ Regen. Med. 1, 16008 (2016).
Fata, J. E., Werb, Z. & Bissell, M. J. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 6, 1–11 (2004).
Huttenlocher, A., Sandborg, R. R. & Horwitz, A. F. Adhesion in cell migration. Curr. Opin. Cell Biol. 7, 697–706 (1995).
Cohen, M., Joester, D., Geiger, B. & Addadi, L. Spatial and temporal sequence of events in cell adhesion: from molecular recognition to focal adhesion assembly. Chembiochem 5, 1393–1399 (2004).
Polanco, T. O., Xylas, J. & Lantis, J. C. II . Extracellular matrices (ECM) for tissue repair. Surg. Technol. Int. 28, 43–57 (2016).
Badylak, S. F., Dziki, J. L., Sicari, B. M., Ambrosio, F. & Boninger, M. L. Mechanisms by which acellular biologic scaffolds promote functional skeletal muscle restoration. Biomaterials 103, 128–136 (2016).
Agrawal, V. et al. An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation. Tissue Eng. Part A 17, 3033–3044 (2011).
Hill, R. C., Calle, E. A., Dzieciatkowska, M., Niklason, L. E. & Hansen, K. C. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol. Cell Proteomics 14, 961–973 (2015).
Calle, E. A. et al. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater. 46, 91–100 (2016).
Li, F. et al. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium 11, 199–206 (2004).
Agrawal, V., Brown, B. N., Beattie, A. J., Gilbert, T. W. & Badylak, S. F. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J. Tissue Eng. Regen. Med. 3, 590–600 (2009).
Davis, G. E., Bayless, K. J., Davis, M. J. & Meininger, G. A. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 156, 1489–1498 (2000).
Huleihel, L. et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2, e1600502 (2016).
Keane, T. J. et al. Tissue-specific effects of esophageal extracellular matrix. Tissue Eng. Part A 21, 2293–2300 (2015).
Wood, L. D. & Montgomery, E. A. in Gastrointestinal Anatomy and Physiology: The Essentials (eds Reinus, J.F. & Simon, D.) 1–14 (John Wiley & Sons, 2014)
Zorn, A. M. & Wells, J. M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251 (2009).
Lebenthal, E. & Lee, P. C. Review article. Interactions of determinants in the ontogeny of the gastrointestinal tract: a unified concept. Pediatr. Res. 17, 19–24 (1983).
Montgomery, R. K., Mulberg, A. E. & Grand, R. J. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 116, 702–731 (1999).
Schoenwolf, G. C., Bleyl, S. B., Brauer, P. R. & Francis-West, P. H. Larsen's Human Embryology 341–374 (Elsevier Health Sciences, 2014).
McLin, V. A., Henning, S. J. & Jamrich, M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology 136, 2074–2091 (2009).
de Santa Barbara, P., van den Brink, G. R. & Roberts, D. J. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. 60, 1322–1332 (2003).
Chiquet-Ehrismann, R., Kalla, P., Pearson, C. A., Beck, K. & Chiquet, M. Tenascin interferes with fibronectin action. Cell 53, 383–390 (1988).
Beaulieu, J. F., Jutras, S., Kusakabe, M. & Perreault, N. Expression of tenascin in the developing human small intestine. Biochem. Biophys. Res. Commun. 192, 1086–1092 (1993).
Beaulieu, J. F., Vachon, P. H. & Chartrand, S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat. Embryol. (Berl.) 183, 363–369 (1991).
Tremblay, E. & Menard, D. Differential expression of extracellular matrix components during the morphogenesis of human gastric mucosa. Anat. Rec. 245, 668–676 (1996).
Menard, D. & Arsenault, P. Cell proliferation in developing human stomach. Anat. Embryol. (Berl.) 182, 509–516 (1990).
Simoneau, A. et al. Identification, distribution, and tissular origin of the alpha5(IV) and alpha6(IV) collagen chains in the developing human intestine. Dev. Dyn. 212, 437–447 (1998).
Simon-Assmann, P., Bouziges, F., Freund, J. N., Perrin-Schmitt, F. & Kedinger, M. Type IV collagen mRNA accumulates in the mesenchymal compartment at early stages of murine developing intestine. J. Cell Biol. 110, 849–857 (1990).
Sato, H. et al. The differential distribution of type IV collagen alpha chains in the subepithelial basement membrane of the human alimentary canal. Arch. Histol. Cytol. 70, 313–323 (2007).
Beaulieu, J. F. et al. Expression of the alpha-5(IV) collagen chain in the fetal human small intestine. Gastroenterology 107, 957–967 (1994).
Khoshnoodi, J., Pedchenko, V. & Hudson, B. G. Mammalian collagen IV. Microsc. Res. Tech. 71, 357–370 (2008).
Leinonen, A., Mariyama, M., Mochizuki, T., Tryggvason, K. & Reeders, S. T. Complete primary structure of the human type IV collagen alpha 4(IV) chain. Comparison with structure and expression of the other alpha (IV) chains. J. Biol. Chem. 269, 26172–26177 (1994).
Wolf, M. T., Daly, K. A., Reing, J. E. & Badylak, S. F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 33, 2916–2925 (2012).
Badylak, S. F. et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J. Biomed. Mater. Res. 29, 977–985 (1995).
Allen, R. A. et al. Adrenal extracellular matrix scaffolds support adrenocortical cell proliferation and function in vitro. Tissue Eng. Part A 16, 3363–3374 (2010).
Sellaro, T. L. et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. Part A 16, 1075–1082 (2010).
Sellaro, T. L., Ravindra, A. K., Stolz, D. B. & Badylak, S. F. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 13, 2301–2310 (2007).
Zhang, Y. et al. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 30, 4021–4028 (2009).
Cortiella, J. et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. Part A 16, 2565–2580 (2010).
Shojaie, S. et al. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: requirement of matrix-bound HS proteoglycans. Stem Cell Rep. 4, 419–430 (2015).
Brennan, E. P., Tang, X. H., Stewart-Akers, A. M., Gudas, L. J. & Badylak, S. F. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J. Tissue Eng. Regen. Med. 2, 491–498 (2008).
Crapo, P. M. et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33, 3539–3547 (2012).
Bhrany, A. D. et al. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng. 12, 319–330 (2006).
Bhrany, A. D. et al. Crosslinking of an oesophagus acellular matrix tissue scaffold. J. Tissue Eng. Regen. Med. 2, 365–372 (2008).
Keane, T. J. et al. Preparation and characterization of a biologic scaffold from esophageal mucosa. Biomaterials 34, 6729–6737 (2013).
Totonelli, G. et al. Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration. Pediatr. Surg. Int. 29, 87–95 (2013).
Badylak, S. F., Lantz, G. C., Coffey, A. & Geddes, L. A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 47, 74–80 (1989).
Maghsoudlou, P., Totonelli, G., Loukogeorgakis, S. P., Eaton, S. & De Coppi, P. A decellularization methodology for the production of a natural acellular intestinal matrix. J. Vis. Exp. 80, e50658 (2013).
Totonelli, G. et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials 33, 3401–3410 (2012).
Lun, S. et al. A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials 31, 4517–4529 (2010).
Floden, E. W. et al. Biophysical characterization of ovine forestomach extracellular matrix biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 96, 67–75 (2011).
Irvine, S. M. et al. Quantification of in vitro and in vivo angiogenesis stimulated by ovine forestomach matrix biomaterial. Biomaterials 32, 6351–6361 (2011).
Parnigotto, P. P., Marzaro, M., Artusi, T., Perrino, G. & Conconi, M. T. Short bowel syndrome: experimental approach to increase intestinal surface in rats by gastric homologous acellular matrix. J. Pediatr. Surg. 35, 1304–1308 (2000).
Sutherland, R. S., Baskin, L. S., Hayward, S. W. & Cunha, G. R. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J. Urol. 156, 571–577 (1996).
Urita, Y. et al. Regeneration of the esophagus using gastric acellular matrix: an experimental study in a rat model. Pediatr. Surg. Int. 23, 21–26 (2007).
Keane, T. J. et al. Preparation and characterization of a biologic scaffold and hydrogel derived from colonic mucosa. J. Biomed. Mater. Res. B Appl. Biomater. 105, 291–306 (2015).
Chen, H. J. et al. A recellularized human colon model identifies cancer driver genes. Nat. Biotechnol. 34, 845–851 (2016).
Yu, J., Peng, S., Luo, D. & March, J. C. In vitro 3D human small intestinal villous model for drug permeability determination. Biotechnol. Bioeng. 109, 2173–2178 (2012).
Costello, C. M. et al. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol. Bioeng. 111, 1222–1232 (2014).
Costello, C. M. et al. 3D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Mol. Pharm. 11, 2030–2039 (2014).
Shaffiey, S. A. et al. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen. Med. 11, 45–61 (2016).
Londono, R. & Badylak, S. F. Regenerative medicine strategies for esophageal repair. Tissue Eng. Part B Rev. 21, 393–410 (2015).
Londono, R., Jobe, B. A., Hoppo, T. & Badylak, S. F. Esophagus and regenerative medicine. World J. Gastroenterol. 18, 6894–6899 (2012).
Strange, P. S. Small intestinal submucosa for laparoscopic repair of large paraesophageal hiatal hernias: a preliminary report. Surg. Technol. Int. 11, 141–143 (2003).
Oelschlager, B. K., Barreca, M., Chang, L. & Pellegrini, C. A. The use of small intestine submucosa in the repair of paraesophageal hernias: initial observations of a new technique. Am. J. Surg. 186, 4–8 (2003).
Lopes, M. F., Cabrita, A., Ilharco, J., Pessa, P. & Patricio, J. Grafts of porcine intestinal submucosa for repair of cervical and abdominal esophageal defects in the rat. J. Invest. Surg. 19, 105–111 (2006).
Badylak, S. F. et al. Esophageal reconstruction with ECM and muscle tissue in a dog model. J. Surg. Res. 128, 87–97 (2005).
Nieponice, A., Gilbert, T. W. & Badylak, S. F. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann. Thorac. Surg. 82, 2050–2058 (2006).
Nieponice, A. et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest. Endosc. 69, 289–296 (2009).
de la Fuente, S. G. et al. Evaluation of porcine-derived small intestine submucosa as a biodegradable graft for gastrointestinal healing. J. Gastrointest. Surg. 7, 96–101 (2003).
Ueno, T. et al. Functional evaluation of the grafted wall with porcine-derived small intestinal submucosa (SIS) to a stomach defect in rats. Surgery 142, 376–383 (2007).
Nishimura, T. et al. In vivo motility evaluation of the grafted gastric wall with small intestinal submucosa. Tissue Eng. Part A 16, 1761–1768 (2010).
Chen, M. K. & Badylak, S. F. Small bowel tissue engineering using small intestinal submucosa as a scaffold. J. Surg. Res. 99, 352–358 (2001).
Shirafkan, A., Montalbano, M., McGuire, J., Rastellini, C. & Cicalese, L. New approaches to increase intestinal length: methods used for intestinal regeneration and bioengineering. World J. Transplant. 6, 1–9 (2016).
Groos, S., Reale, E., Hunefeld, G. & Luciano, L. Changes in epithelial cell turnover and extracellular matrix in human small intestine after TPN. J. Surg. Res. 109, 74–85 (2003).
Wang, Z. Q., Watanabe, Y. & Toki, A. Experimental assessment of small intestinal submucosa as a small bowel graft in a rat model. J. Pediatr. Surg. 38, 1596–1601 (2003).
Wang, Z. Q. et al. Morphologic evaluation of regenerated small bowel by small intestinal submucosa. J. Pediatr. Surg. 40, 1898–1902 (2005).
Ansaloni, L. et al. Experimental evaluation of Surgisis as scaffold for neointestine regeneration in a rat model. Transplant. Proc. 38, 1844–1848 (2006).
Demirbilek, S., Kanmaz, T., Ozardali, I., Edali, M. N. & Yucesan, S. Using porcine small intestinal submucosa in intestinal regeneration. Pediatr. Surg. Int. 19, 588–592 (2003).
Lee, M., Chang, P. C. & Dunn, J. C. Evaluation of small intestinal submucosa as scaffolds for intestinal tissue engineering. J. Surg. Res. 147, 168–171 (2008).
Qin, H. H. & Dunn, J. C. Small intestinal submucosa seeded with intestinal smooth muscle cells in a rodent jejunal interposition model. J. Surg. Res. 171, e21–e26 (2011).
Cintron, J. R. et al. Treatment of fistula-in-ano using a porcine small intestinal submucosa anal fistula plug. Tech. Coloproctol. 17, 187–191 (2013).
Schultz, D. J., Brasel, K. J., Spinelli, K. S., Rasmussen, J. & Weigelt, J. A. Porcine small intestine submucosa as a treatment for enterocutaneous fistulas. J. Am. Coll. Surg. 194, 541–543 (2002).
Ueno, T., Oga, A., Takahashi, T. & Pappas, T. N. Small intestinal submucosa (SIS) in the repair of a cecal wound in unprepared bowel in rats. J. Gastrointest. Surg. 11, 918–922 (2007).
Keane, T. J. et al. Restoring mucosal barrier function and modifying macrophage phenotype with an extracellular matrix hydrogel: potential therapy for ulcerative colitis. J. Crohns Colitis (2016).
Hoshiba, T. et al. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016, 6397820 (2016).
Hoshiba, T. & Tanaka, M. Decellularized matrices as in vitro models of extracellular matrix in tumor tissues at different malignant levels: mechanism of 5-fluorouracil resistance in colorectal tumor cells. Biochim. Biophys. Acta 1863, 2749–2757 (2016).
Ozeki, M. et al. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J. Biomed. Mater. Res. A 79, 771–778 (2006).
De Waele, J. et al. 3D culture of murine neural stem cells on decellularized mouse brain sections. Biomaterials 41, 122–131 (2015).
Ingber, D. E. Can cancer be reversed by engineering the tumor microenvironment? Semin. Cancer Biol. 18, 356–364 (2008).
Nyga, A., Cheema, U. & Loizidou, M. 3D tumour models: novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 5, 239–248 (2011).
Genovese, L. et al. Cellular localization, invasion, and turnover are differently influenced by healthy and tumor-derived extracellular matrix. Tissue Eng. Part A 20, 2005–2018 (2014).
Hosseinkhani, M. et al. Tissue engineered scaffolds in regenerative medicine. World J. Plast. Surg. 3, 3–7 (2014).
Gjorevski, N., Ranga, A. & Lutolf, M. P. Bioengineering approaches to guide stem cell-based organogenesis. Development 141, 1794–1804 (2014).
Ahmed, M. & Ffrench-Constant, C. Extracellular matrix regulation of stem cell behavior. Curr. Stem Cell Rep. 2, 197–206 (2016).
Bitar, K. N. & Zakhem, E. Bioengineering the gut: future prospects of regenerative medicine. Nat. Rev. Gastroenterol. Hepatol. 13, 543–556 (2016).
Pentinmikko, N. & Katajisto, P. Microenvironment directs the life of stem cells [Finnish]. Duodecim 130, 1965–1972 (2014).
Naba, A. et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteomics 11, M111.014647 (2012).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Decellularization
-
A process used in tissue engineering to remove cells from a tissue or an organ, without affecting the extracellular matrix of the original tissue.
- Stricture
-
Narrowing of a tube or channel.
- Xenogeneic
-
Tissues or cells belonging to individuals of different species.
- Allogeneic
-
Tissues or cells from the same species that are genetically dissimilar and immunologically incompatible.
- Anorectal fistulas
-
Small tracts that develop between the end of the bowel and the skin near the anus.
- Anastomosis
-
A cross-connection between adjacent channels of the body.
- Matricryptic peptides
-
Hidden peptide residues in collagen molecules that are released after the degradation of the parent molecule.
- Caustic oesophagitis
-
Injury to the oesophagus due to the ingestion of a strong alkali or acid.
- Barrett oesophagus
-
A condition in which the cells of the oesophagus grow abnormally.
- Oesophageal stenosis
-
An abnormal narrowing of the oesophagus.
- Oesophageal dilatation
-
Stretching or widening of the oesophagus.
- Oesophagoplasty
-
Plastic surgery for the repair or reconstruction of the oesophagus.
- Diverticula
-
A protrusion of a hollow (or a fluid-filled) structure in the body.
- Oesophageal transection
-
The removal of the lower end of the oesophagus with end-to-end anastomosis.
- Ileostomy
-
A surgical procedure in which a piece of the ileum is diverted to an opening in the abdominal wall.
- Celiotomy
-
A surgical procedure that involves a large incision through the abdominal wall to gain access to the abdominal cavity.
- Onlay mesh repair
-
Use of a mesh material placed over a defect to repair an incision.
- Matrisome
-
The ensemble of extracellular matrix (ECM) and ECM-associated proteins.
Rights and permissions
About this article
Cite this article
Hussey, G., Keane, T. & Badylak, S. The extracellular matrix of the gastrointestinal tract: a regenerative medicine platform. Nat Rev Gastroenterol Hepatol 14, 540–552 (2017). https://doi.org/10.1038/nrgastro.2017.76
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.76
This article is cited by
-
Regenerative medicine: current research and perspective in pediatric surgery
Pediatric Surgery International (2023)
-
Application of decellularized bone matrix as a bioscaffold in bone tissue engineering
Journal of Biological Engineering (2022)
-
A Serological Biomarker of Laminin Gamma 1 Chain Degradation Reflects Altered Basement Membrane Remodeling in Crohn’s Disease and DSS Colitis
Digestive Diseases and Sciences (2022)
-
Novel Marine Organism-Derived Extracellular Vesicles for Control of Anti-Inflammation
Tissue Engineering and Regenerative Medicine (2021)
-
Development of immortalized Hertwig’s epithelial root sheath cell lines for cementum and dentin regeneration
Stem Cell Research & Therapy (2019)