Key Points
-
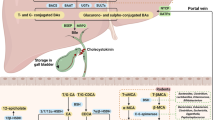
The discovery of dedicated bile acid receptors and further research defined multiple bile acid signalling routes affecting bile acid synthesis, lipid synthesis, gluconeogenesis, inflammation, liver fibrosis and cancer
-
Steroidal and nonsteroidal agonists of bile acid receptors have been developed as potential treatments for cholestatic and metabolic liver diseases, including primary biliary cirrhosis, primary sclerosing cholangitis and NASH
-
Randomized, placebo-controlled clinical trials for the treatment of primary biliary cirrhosis and NASH with the farnesoid X receptor (FXR) agonist obeticholic acid are the first clinical trials initiated
-
Future indications for FXR and TGR5 agonists include genetic cholestatic syndromes, intrahepatic cholestasis of pregnancy, atherosclerosis, IBS, bile-reflux oesophagitis, IBD, hepatocellular and colon carcinoma
Abstract
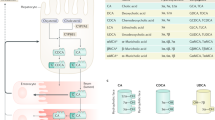
The intracellular nuclear receptor farnesoid X receptor and the transmembrane G protein-coupled receptor TGR5 respond to bile acids by activating transcriptional networks and/or signalling cascades. These cascades affect the expression of a great number of target genes relevant for bile acid, cholesterol, lipid and carbohydrate metabolism, as well as genes involved in inflammation, fibrosis and carcinogenesis. Pregnane X receptor, vitamin D receptor and constitutive androstane receptor are additional nuclear receptors that respond to bile acids, albeit to a more restricted set of species of bile acids. Recognition of dedicated bile acid receptors prompted the development of semi-synthetic bile acid analogues and nonsteroidal compounds that target these receptors. These agents hold promise to become a new class of drugs for the treatment of chronic liver disease, hepatocellular cancer and extrahepatic inflammatory and metabolic diseases. This Review discusses the relevant bile acid receptors, the new drugs that target bile acid signalling and their possible applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hagey, L. R. et al. Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores. J. Lipid Res. 34, 1911–1917 (1993).
Maton, P. N., Murphy, G. M. & Dowling, R. H. Ursodeoxycholic acid treatment of gallstones. Dose-response study and possible mechanism of action. Lancet 2, 1297–1301 (1977).
Poupon, R. et al. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet 1, 834–836 (1987).
Salen, G., Meriwether, T. W. & Nicolau, G. Chenodeoxycholic acid inhibits increased cholesterol and cholestanol synthesis in patients with cerebrotendinous xanthomatosis. Biochem. Med. 14, 57–74 (1975).
Hench, P. S. Effect of jaundice on rheumatoid arthritis. Br. Med. J. 2, 394–398 (1938).
Russell, D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 (2003).
Ridlon, J. M., Kang, D. J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Dawson, P. A., Lan, T. & Rao, A. Bile acid transporters. J. Lipid Res. 50, 2340–2357 (2009).
Hofmann, A. F. Biliary secretion and excretion in health and disease: current concepts. Ann. Hepatol. 6, 15–27 (2007).
Borgstrom, B., Dahlqvist, A., Lundh, G. & Sjovall, J. Studies of intestinal digestion and absorption in the human. J. Clin. Invest. 36, 1521–1536 (1957).
Forman, B. M. et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81, 687–693 (1995).
Seol, W., Choi, H. S. & Moore, D. D. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol. Endocrinol. 9, 72–85 (1995).
Shefer, S., Hauser, S., Bekersky, I. & Mosbach, E. H. Feedback regulation of bile acid biosynthesis in the rat. J. Lipid Res. 10, 646–655 (1969).
Goodwin, B. et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6, 517–526 (2000).
Seol, W., Choi, H. S. & Moore, D. D. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272, 1336–1339 (1996).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell. Metab. 2, 217–225 (2005).
Houten, S. M., Watanabe, M. & Auwerx, J. Endocrine functions of bile acids. EMBO J. 25, 1419–1425 (2006).
Huang, W. et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312, 233–236 (2006).
Inagaki, T. et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl Acad. Sci. USA 103, 3920–3925 (2006).
Alemi, F. et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144, 145–154 (2013).
Higuchi, H., Grambihler, A., Canbay, A., Bronk, S. F. & Gores, G. J. Bile acids up-regulate death receptor 5/TRAIL-receptor 2 expression via a c-Jun N.-terminal kinase-dependent pathway involving Sp1. J. Biol. Chem. 279, 51–60 (2004).
Allen, K., Jaeschke, H. & Copple, B. L. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 178, 175–186 (2011).
Baes, M. et al. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol. Cell Biol. 14, 1544–1552 (1994).
Makishima, M. et al. Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002).
Choi, H. S. et al. Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J. Biol. Chem. 272, 23565–23571 (1997).
Maruyama, T. et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298, 714–719 (2002).
Kawamata, Y. et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 (2003).
Otte, K. et al. Identification of farnesoid X receptor beta as a novel mammalian nuclear receptor sensing lanosterol. Mol. Cell Biol. 23, 864–872 (2003).
Zhang, Y., Kast-Woelbern, H. R. & Edwards, P. A. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J. Biol. Chem. 278, 104–110 (2003).
Bookout, A. L. et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126, 789–799 (2006).
Li, Z., Kruijt, J. K., van der Sluis, R. J., Van Berkel, T. J. & Hoekstra, M. Nuclear receptor atlas of female mouse liver parenchymal, endothelial, and Kupffer cells. Physiol. Genomics 45, 268–275 (2013).
Fickert, P. et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am. J. Pathol. 175, 2392–2405 (2009).
Bishop-Bailey, D., Walsh, D. T. & Warner, T. D. Expression and activation of the farnesoid X receptor in the vasculature. Proc. Natl Acad. Sci. USA 101, 3668–3673 (2004).
Lee, H. et al. FXR regulates organic solute transporters α and β in the adrenal gland, kidney, and intestine. J. Lipid Res. 47, 201–214 (2006).
Cariou, B. et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem. 281, 11039–11049 (2006).
Jiang, T. et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 (2007).
Abel, U. et al. Synthesis and pharmacological validation of a novel series of non-steroidal FXR agonists. Bioorg. Med. Chem. Lett. 20, 4911–4917 (2010).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell. Metab. 17, 225–235 (2013).
Beraza, N. et al. Nor-ursodeoxycholic acid reverses hepatocyte-specific nemo-dependent steatohepatitis. Gut 60, 387–396 (2011).
Gardmo, C., Tamburro, A., Modica, S. & Moschetta, A. Proteomics for the discovery of nuclear bile acid receptor FXR targets. Biochim. Biophys. Acta 1812, 836–841 (2011).
Jung, D. & Kullak-Ublick, G. A. Hepatocyte nuclear factor 1 α: a key mediator of the effect of bile acids on gene expression. Hepatology 37, 622–631 (2003).
Jung, D., Mangelsdorf, D. J. & Meyer, U. A. Pregnane X receptor is a target of farnesoid X receptor. J. Biol. Chem. 281, 19081–19091 (2006).
Inoue, J. et al. PPARα gene expression is up-regulated by LXR and PXR activators in the small intestine. Biochem. Biophys. Res. Commun. 371, 675–678 (2008).
Renga, B. et al. Farnesoid X receptor suppresses constitutive androstane receptor activity at the multidrug resistance protein-4 promoter. Biochim. Biophys. Acta 1809, 157–165 (2011).
Beilke, L. D. et al. Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice. Drug Metab. Dispos. 37, 1035–1045 (2009).
Claudel, T., Staels, B. & Kuipers, F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler. Thromb. Vasc. Biol. 25, 2020–2030 (2005).
Wang, Y. D., Chen, W. D., Moore, D. D. & Huang, W. FXR: a metabolic regulator and cell protector. Cell Res. 18, 1087–1095 (2008).
Pircher, P. C. et al. Farnesoid X receptor regulates bile acid-amino acid conjugation. J. Biol. Chem. 278, 27703–27711 (2003).
Ananthanarayanan, M., Balasubramanian, N., Makishima, M., Mangelsdorf, D. J. & Suchy, F. J. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 276, 28857–28865 (2001).
Boyer, J. L. et al. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTα-OSTβ in cholestasis in humans and rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1124–G1130 (2006).
Fiorucci, S. et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 127, 1497–1512 (2004).
Kim, I. et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28, 940–946 (2007).
Deuschle, U. et al. FXR controls the tumor suppressor NDRG2 and FXR agonists reduce liver tumor growth and metastasis in an orthotopic mouse xenograft model. PLoS ONE 7, e43044 (2012).
Jiang, Y. et al. Farnesoid X receptor inhibits gankyrin in mouse livers and prevents development of liver cancer. Hepatology 57, 1098–1106 (2013).
Wistuba, W., Gnewuch, C., Liebisch, G., Schmitz, G. & Langmann, T. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR. World J. Gastroenterol. 13, 4230–4235 (2007).
Schmidt, D. R. et al. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem. 285, 14486–14494 (2010).
Schreuder, T. C. et al. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am. J. Physiol. Gastrointest Liver Physiol. 298, G440–G445 (2010).
Triantis, V., Saeland, E., Bijl, N., Oude-Elferink, R. P. & Jansen, P. L. Glycosylation of fibroblast growth factor receptor 4 is a key regulator of fibroblast growth factor 19-mediated down-regulation of cytochrome P450 7A1. Hepatology 52, 656–666 (2010).
Wu, X. et al. Co-receptor requirements for fibroblast growth factor-19 signaling. J. Biol. Chem. 282, 29069–29072 (2007).
Kir, S., Zhang, Y., Gerard, R. D., Kliewer, S. A. & Mangelsdorf, D. J. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 287, 41334–41341 (2012).
Kim, I. et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 48, 2664–2672 (2007).
Potthoff, M. J. et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell. Metab. 13, 729–738 (2011).
Kir, S. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011).
Miyata, M., Sakaida, Y., Matsuzawa, H., Yoshinari, K. & Yamazoe, Y. Fibroblast growth factor 19 treatment ameliorates disruption of hepatic lipid metabolism in farnesoid X receptor (Fxr)-null mice. Biol. Pharm. Bull. 34, 1885–1889 (2011).
Choi, M. et al. Identification of a hormonal basis for gallbladder filling. Nat. Med. 12, 1253–1255 (2006).
Drafahl, K. A., McAndrew, C. W., Meyer, A. N., Haas, M. & Donoghue, D. J. The receptor tyrosine kinase FGFR4 negatively regulates NF-κB signaling. PLoS ONE 5, e14412 (2010).
Uriarte, I. et al. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut 62, 899–910 (2013).
Zweers, S. J. et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 55, 575–583 (2012).
Nicholes, K. et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 160, 2295–2307 (2002).
Lin, B. C. & Desnoyers, L. R. FGF19 and cancer. Adv. Exp. Med. Biol. 728, 183–194 (2012).
Wu, X. et al. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19). Proc. Natl Acad. Sci. USA 107, 14158–14163 (2010).
Keitel, V. et al. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 50, 861–870 (2009).
Keitel, V. et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58, 1794–1805 (2010).
Keitel, V. et al. The G.-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology 45, 695–704 (2007).
Sato, H. et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 362, 793–798 (2007).
Sato, H. et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 51, 1831–1841 (2008).
Pols, T. W., Noriega, L. G., Nomura, M., Auwerx, J. & Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 54, 1263–1272 (2011).
Pols, T. W. et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell. Metab. 14, 747–757 (2011).
Wang, Y. D., Chen, W. D., Yu, D., Forman, B. M. & Huang, W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 54, 1421–1432 (2011).
Katsuma, S., Hirasawa, A. & Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 329, 386–390 (2005).
Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell. Metab. 10, 167–177 (2009).
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Jansen, P. L. et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig. Dis. 29, 48–51 (2011).
Li, T. et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol. Endocrinol. 25, 1066–1071 (2011).
Beuers, U. et al. The biliary HCO3− umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 52, 1489–1496 (2010).
Zhang, H. et al. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch. Biochem. Biophys. 368, 14–22 (1999).
Kliewer, S. A. et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92, 73–82 (1998).
Lehmann, J. M. et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 102, 1016–1023 (1998).
Bertilsson, G. et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc. Natl Acad. Sci. USA 95, 12208–12213 (1998).
Lamba, V. et al. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol. Appl. Pharmacol. 199, 251–265 (2004).
Vogel, S. M. et al. Lithocholic acid is an endogenous inhibitor of MDM4 and MDM2. Proc. Natl Acad. Sci. USA 109, 16906–16910 (2012).
Wang, Y. M., Ong, S. S., Chai, S. C. & Chen, T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin. Drug Metab. Toxicol. 8, 803–817 (2012).
Kast, H. R. et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 277, 2908–2915 (2002).
Ihunnah, C. A., Jiang, M. & Xie, W. Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim. Biophys. Acta 1812, 956–963 (2011).
Wallace, K. et al. The PXR is a drug target for chronic inflammatory liver disease. J. Steroid Biochem. Mol. Biol. 120, 137–148 (2010).
Li, T. & Chiang, J. Y. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 α-hydroxylase gene transcription. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G74–G84 (2005).
Cheng, J., Shah, Y. M. & Gonzalez, F. J. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol. Sci. 33, 323–330 (2012).
Zhou, J. et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARγ in promoting steatosis. Gastroenterology 134, 556–567 (2008).
Jonker, J. W., Liddle, C. & Downes, M. FXR and PXR: potential therapeutic targets in cholestasis. J. Steroid Biochem. Mol. Biol. 130, 147–158 (2012).
Norman, A. W. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147, 5542–5548 (2006).
Han, S. & Chiang, J. Y. Mechanism of vitamin D receptor inhibition of cholesterol 7α-hydroxylase gene transcription in human hepatocytes. Drug Metab. Dispos. 37, 469–478 (2009).
Adachi, R. et al. Structural determinants for vitamin D receptor response to endocrine and xenobiotic signals. Mol. Endocrinol. 18, 43–52 (2004).
Huhtakangas, J. A., Olivera, C. J., Bishop, J. E., Zanello, L. P. & Norman, A. W. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3 in vivo and in vitro. Mol. Endocrinol. 18, 2660–2671 (2004).
Han, S., Li, T., Ellis, E., Strom, S. & Chiang, J. Y. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol. Endocrinol. 24, 1151–1164 (2010).
D'Aldebert, E. et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology 136, 1435–1443 (2009).
Saini, S. P. et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol. Pharmacol. 65, 292–300 (2004).
Chang, T. K. Activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by herbal medicines. AAPS J. 11, 590–601 (2009).
Moore, L. B. et al. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol. Endocrinol. 16, 977–986 (2002).
Miao, J., Fang, S., Bae, Y. & Kemper, J. K. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1α. J. Biol. Chem. 281, 14537–14546 (2006).
Uppal, H. et al. Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology 41, 168–176 (2005).
Pellicciari, R. et al. 6α-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 45, 3569–3572 (2002).
Pellicciari, R. et al. Discovery of 6α-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 52, 7958–7961 (2009).
Rizzo, G. et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol. Pharmacol. 78, 617–630 (2010).
Baghdasaryan, A. et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO3− output. Hepatology 54, 1303–1312 (2011).
Adorini, L., Pruzanski, M. & Shapiro, D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov. Today 17, 988–997 (2012).
Hartman, H. B. et al. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J. Lipid Res. 50, 1090–1100 (2009).
Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 296, H272–H281 (2009).
Urizar, N. L. et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296, 1703–1706 (2002).
Zhang, Y. et al. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler Thromb. Vasc Biol. 26, 2316–2321 (2006).
Guo, G. L. et al. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta 1761, 1401–1409 (2006).
Bennett, B. J. et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell. Metab. 17, 49–60 (2013).
Marinozzi, M. et al. Pyrazole[3,4-e][1 4]thiazepin-7-one derivatives as a novel class of farnesoid X receptor (FXR) agonists. Bioorg. Med. Chem. 20, 3429–3445 (2012).
Herbert, M. R. et al. Synthesis and SAR of 2-aryl-3-aminomethylquinolines as agonists of the bile acid receptor TGR5. Bioorg. Med. Chem. Lett. 20, 5718–5721 (2010).
Sakamoto, S. et al. Glucuronidation converting methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6, 7,8-trimethoxy-2-naphthoat e (S-8921) to a potent apical sodium-dependent bile acid transporter inhibitor, resulting in a hypocholesterolemic action. J. Pharmacol. Exp. Ther. 322, 610–618 (2007).
Kobayashi, M. et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes 56, 239–247 (2007).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J. Hepatol. 51, 237–267 (2009).
Beuers, U. et al. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology 33, 1206–1216 (2001).
Garcia-Marin, J. J., Dumont, M., Corbic, M., de Couet, G. & Erlinger, S. Effect of acid-base balance and acetazolamide on ursodeoxycholate-induced biliary bicarbonate secretion. Am. J. Physiol. 248, G20–G27 (1985).
Kurz, A. K., Graf, D., Schmitt, M., Vom Dahl, S. & Haussinger, D. Tauroursodesoxycholate-induced choleresis involves p38(MAPK) activation and translocation of the bile salt export pump in rats. Gastroenterology 121, 407–419 (2001).
Gohlke, H., Schmitz, B., Sommerfeld, A., Reinehr, R. & Haussinger, D. α5 β1-integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology 57, 1117–1129 (2013).
Bouscarel, B., Fromm, H. & Nussbaum, R. Ursodeoxycholate mobilizes intracellular Ca2+ and activates phosphorylase a in isolated hepatocytes. Am. J. Physiol. 264, G243–G251 (1993).
Wong, M. H., Oelkers, P., Craddock, A. L. & Dawson, P. A. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J. Biol. Chem. 269, 1340–1347 (1994).
Mekjian, H. S., Phillips, S. F. & Hofmann, A. F. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J. Clin. Invest. 50, 1569–1577 (1971).
Harach, T. et al. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci. Rep. 2, 430 (2012).
Chen, L. et al. Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am. J. Physiol. Endocrinol. Metab. 302, E68–E76 (2012).
Bhat, B. G. et al. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J. Lipid Res. 44, 1614–1621 (2003).
Li, H. et al. Inhibition of ileal bile acid transport lowers plasma cholesterol levels by inactivating hepatic farnesoid X receptor and stimulating cholesterol 7 α-hydroxylase. Metabolism 53, 927–932 (2004).
Insull, W. Jr et al. Effectiveness of colesevelam hydrochloride in decreasing LDL cholesterol in patients with primary hypercholesterolemia: a 24-week randomized controlled trial. Mayo Clin. Proc. 76, 971–982 (2001).
Hansen, M. et al. Effect of bile acid sequestrants on glycaemic control: protocol for a systematic review with meta-analysis of randomised controlled trials. BMJ Open 2, e001803 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Schaap, F. G., van der Gaag, N. A., Gouma, D. J. & Jansen, P. L. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 49, 1228–1235 (2009).
Fickert, P. et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127, 261–274 (2004).
Fiorucci, S. et al. Farnesoid X receptor agonist for the treatment of liver and metabolic disorders: focus on 6-ethyl-CDCA. Mini Rev. Med. Chem. 11, 753–762 (2011).
Kremer, A. E. et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 139, 1008–1018 (2010).
Kremer, A. E. et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology 56, 1391–1400 (2012).
Alemi, F. et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J. Clin. Invest. 123, 1513–1530 (2013).
Gadaleta, R. M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).
Terjung, B. et al. p-ANCAs in autoimmune liver disorders recognise human β-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut 59, 808–816 (2010).
Wong, B. S. et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin. Gastroenterol. Hepatol. 10, 1009–1015 e3 (2012).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology http://dx.doi.org/10.1053/j.gastro.2013.05.042.
Wang, X. X. et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59, 2916–2927 (2010).
Peterli, R. et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann. Surg. 250, 234–241 (2009).
van Dijk, R., Kremer, A. F., Enemuo, V., Jansen, P. L., Beuers, U. Clinical and molecular aspects of rifampicin-mediated attenuation of severe persistent hepatocellular secretory failure. Hepatology 56, 549A (2012).
Ohkubo, H., Okuda, K. & Iida, S. Effects of corticosteroids on bilirubin metabolism in patients with Gilbert's syndrome. Hepatology 1, 168–172 (1981).
Arias, I. M., Gartner, L. M., Cohen, M., Ezzer, J. B. & Levi, A. J. Chronic nonhemolytic unconjugated hyperbilirubinemia with glucuronyl transferase deficiency. Clinical, biochemical, pharmacologic and genetic evidence for heterogeneity. Am. J. Med. 47, 395–409 (1969).
Ellis, E. et al. Successful treatment of severe unconjugated hyperbilirubinemia via induction of UGT1A1 by rifampicin. J. Hepatol. 44, 243–245 (2006).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Downes, M. et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol. Cell 11, 1079–1092 (2003).
Hambruch, E. et al. Synthetic farnesoid X receptor agonists induce high-density lipoprotein-mediated transhepatic cholesterol efflux in mice and monkeys and prevent atherosclerosis in cholesteryl ester transfer protein transgenic low-density lipoprotein receptor(−/−) mice. J. Pharmacol. Exp. Ther. 343, 556–567 (2012).
Flatt, B. et al. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR). J. Med. Chem. 52, 904–907 (2009).
Soisson, S. M. et al. Identification of a potent synthetic FXR agonist with an unexpected mode of binding and activation. Proc. Natl Acad. Sci. USA 105, 5337–5342 (2008).
Bass, J. Y. et al. Conformationally constrained farnesoid X receptor (FXR) agonists: heteroaryl replacements of the naphthalene. Bioorg. Med. Chem. Lett. 21, 1206–1213 (2011).
Chen, W. D. et al. Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription. Hepatology 51, 953–962 (2010).
Hollman, D. A., Milona, A., van Erpecum, K. J. & van Mil, S. W. Anti-inflammatory and metabolic actions of FXR: insights into molecular mechanisms. Biochim. Biophys. Acta 1821, 1443–1452 (2012).
Staudinger, J. L. et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA 98, 3369–3374 (2001).
Jones, S. A. et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol. Endocrinol. 14, 27–39 (2000).
Moore, L. B. et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl Acad. Sci. USA 97, 7500–7502 (2000).
Ogura, M. et al. Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. J. Pharmacol. Exp. Ther. 328, 564–570 (2009).
Ma, Y. et al. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J. Clin. Invest. 116, 892–904 (2006).
Maglich, J. M. et al. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 62, 638–646 (2002).
Gao, J. & Xie, W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol. Sci. 33, 552–558 (2012).
Acknowledgements
The authors of this Review are supported by grantsP19118-B05, F3008 and F3517-B20 from the Austrian Science Foundation and European Community's Seventh Framework Program (FP7/2007-2013) under grant agreement HEALTH-F2-2009-241762 for the project FLIP (M. Trauner) and the Dutch Digestive Diseases Foundation (MLDS WO 08-69; to P. L. M. Jansen and F. G. Schaap).
Author information
Authors and Affiliations
Contributions
P. L. M. Jansen and F. G. Schaap wrote the article. M. Trauner reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. Trauner has received unrestricted research grants from Falk Pharma and Intercept and acts as an advisor for Phenex. The Medical University of Graz has filed a patent on the medical use of norUDCA and M. Trauner is listed as co-inventor. F. G. Schaap and P. L. M. Jensen declare no competing interests.
Rights and permissions
About this article
Cite this article
Schaap, F., Trauner, M. & Jansen, P. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 11, 55–67 (2014). https://doi.org/10.1038/nrgastro.2013.151
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.151
This article is cited by
-
Roles of bile acids signaling in neuromodulation under physiological and pathological conditions
Cell & Bioscience (2023)
-
Gut microbial metabolite deoxycholic acid facilitates Th17 differentiation through modulating cholesterol biosynthesis and participates in high-fat diet-associated colonic inflammation
Cell & Bioscience (2023)
-
Gut–liver axis: barriers and functional circuits
Nature Reviews Gastroenterology & Hepatology (2023)
-
A pilot metabolomic study of drug interaction with the immune response to seasonal influenza vaccination
npj Vaccines (2023)
-
Influence of cholestasis on portal vein embolization-induced hypertrophy of the future liver remnant
Langenbeck's Archives of Surgery (2023)