Abstract
This Review covers the rationale, physiological consequences and clinical application of pharmacological sodium–glucose cotransporter 2 (SGLT2) inhibition. In patients with type 2 diabetes mellitus, in whom renal glucose reabsorption might be upregulated, orally active, selective SGLT2 inhibitors improve glycaemic control to a therapeutically useful extent. Chronic administration of several SGLT2 inhibitors dose-dependently lowers HbA1c levels by 0.5–1.5% without causing hypoglycaemia. The unique mechanism of action of SGLT2 inhibitors—which does not hinge upon β-cell function or tissue insulin sensitivity—means that they can exert their antihyperglycaemic effects in combination with any other oral antidiabetic drug as well as insulin. Available phase III studies confirm a good tolerability profile. Weight loss owing to urinary calorie leakage may be less than expected, but the negative energy balance offers a valuable clinical benefit. Offloading of sodium can assist blood pressure control. The progressive loss of efficacy in patients with reduced glomerular function will have to be balanced against the possibility of renal protection. The safety issues of genitourinary infections and cancer risk requires careful, proactive monitoring and analysis of robust exposure data, particularly in elderly, frail patients and in patients with impaired kidney function and/or high cardiovascular/cancer risk, who represent an increasing fraction of the population with diabetes mellitus.
Key Points
-
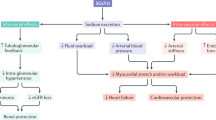
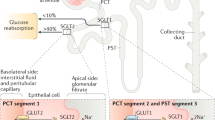
Sodium–glucose cotransporter 2 (SGLT2) is the major cotransporter involved in reabsorption of filtered glucose in the tubular nephron of the kidney
-
Chronic hyperglycaemia in patients with diabetes mellitus might upregulate glucose reabsorption in the kidneys above normal levels
-
SGLT2 inhibitors lower the threshold for glycosuria by lowering the maximum transport capacity or, more likely, by reducing the affinity of the transporter for glucose without causing hypoglycaemia
-
SGLT2 inhibitors cause proximal diuresis and calorie leakage into the urine; therefore, the benefits of these agents could include blood pressure lowering and weight control
-
Increased risk of genitourinary infections is a consistent adverse effect of SGLT2 inhibitors
-
Gauging the effect of SGLT2 inhibitors in the population at high risk for kidney failure and cancer will require analysis of robust exposure data in large populations
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
DeFronzo, R. A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009).
Meier, J. J., Ueberberg, S., Korbas, S. & Schneider, S. Diminished glucagon suppression after β-cell reduction is due to impaired α-cell function rather than an expansion of α-cell mass. Am. J. Physiol. Endocrinol. Metab. 300, E717–E723 (2011).
Ferrannini, E. The stunned beta cell: a brief history. Cell Metab. 11, 349–352 (2010).
Schwanstecher, C. & Schwanstecher, M. Targeting type 2 diabetes. Handb. Exp. Pharmacol. 203, 1–33 (2011).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Kaneto, H., Katakami, N., Matsuhisa, M. & Matsuoka, T. A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010, 43892 (2010).
Jonas, J. C. et al. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes. Metab. 11 (Suppl. 4), 65–81 (2009).
Leibowitz, G. et al. Glucose regulation of β-cell stress in type 2 diabetes. Diabetes Obes. Metab. 12 (Suppl. 2), 66–75 (2010).
Wright, E. M., Loo, D. D. & Hirayama, B. A. Biology of human sodium glucose transporters. Physiol. Rev. 91, 733–794 (2011).
Hummel, C. S. et al. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am. J. Physiol. Cell Physiol. 300, C14–C21 (2011).
Banerjee, S. K. et al. SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J. Mol. Cell. Cardiol. 49, 683–692 (2010).
Li, H., Gu, Y., Zhang, Y., Lucas, M. J. & Wang, Y. High glucose levels down-regulate glucose transporter expression that correlates with increased oxidative stress in placental trophoblast cells in vitro. J. Soc. Gynecol. Investig. 11, 75–81 (2004).
von Lewinski, D. et al. Glucose-transporter-mediated positive inotropic effects in human myocardium of diabetic and nondiabetic patients. Metabolism 59, 1020–1028 (2010).
Takata, K. Glucose transporters in the transepithelial transport of glucose. J. Electron Microsc. 45, 275–284 (1996).
Vallon, V. et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 22, 104–112 (2011).
Wright, E. M., Hirayama, B. A. & Loo, D. F. Active sugar transport in health and disease. J. Intern. Med. 261, 32–43 (2007).
Ruhnau, B., Faber, O. K., Borch-Johnsen, K. & Thorsteinsson, B. Renal threshold for glucose in non-insulin-dependent diabetic patients. Diabetes Res. Clin. Pract. 36, 27–33 (1997).
Farber, S. J., Berger, E. Y. & Earle, D. P. Effect of diabetes and insulin on the maximum capacity of the renal tubules to reabsorb glucose. J. Clin. Invest. 30, 125–129 (1951).
Elsas, L. J. & Rosenberg, L. E. Familial renal glycosuria: a genetic reappraisal of hexose transport by kidney and intestine. J. Clin. Invest. 48, 1845–1854 (1969).
Sha, S. et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 13, 669–672 (2011).
Maurer, T. S. et al. Pharmacodynamic model of sodium-glucose transporter 2 (SGLT2) inhibition: implications for quantitative translational pharmacology. AAPS J. 13, 576–584 (2011).
Mogensen, C. E. Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand. J. Clin. Lab. Invest. 28, 101–109 (1971).
Rahmoune, H. et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54, 3427–3434 (2005).
Jurczak, M. J. et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 60, 890–898 (2011).
Pontoglio, M. et al. HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 1, 359–365 (2000).
Yamagata, K. et al. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature 384, 455–458 (1996).
Schmidt, C., Höcherl, K. & Bucher, M. Regulation of renal glucose transporters during severe inflammation. Am. J. Physiol. Renal Physiol. 292, F804–F811 (2007).
Bautista, R. et al. Angiotensin II-dependent increased expression of Na+-glucose cotransporter in hypertension. Am. J. Physiol. Renal Physiol. 286, F127–F133 (2004).
Ly, J. P. et al. The Sweet Pee model for Sglt2 mutation. J. Am. Soc. Nephrol. 22, 113–123 (2011).
Wright, E. M., Turk, E. & Martin, M. G. Molecular basis for glucose-galactose malabsorption. Cell Biochem. Biophys. 36, 115–121 (2002).
Calado, J., Soto, K., Clemente, C., Correia, P. & Rueff, J. Novel compound heterozygous mutations in SLC5A2 are responsible for autosomal recessive renal glucosuria. Hum. Genet. 114, 314–316 (2004).
Calado, J. et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol. Dial. Transplant. 23, 3874–3879 (2008).
Calado, J. et al. Familial renal glucosuria: SLC5A2 mutation analysis and evidence of salt-wasting. Kidney Int. 69, 852–855 (2006).
Oemar, B. S., Byrd, D. J. & Brodehl, J. Complete absence of tubular glucose reabsorption: a new type of renal glucosuria (type 0). Clin. Nephrol. 27, 156–160 (1987).
Gopalakrishnan, A., Kumar, M., Krishnamurthy, S., Sakamoto, O. & Srinivasan, S. Fanconi-Bickel syndrome in a 3-year-old Indian boy with a novel mutation in the GLUT2 gene. Clin. Exp. Nephrol. 15, 745–748 (2011).
Ehrenkranz, J. R., Lewis, N. G., Kahn, C. R. & Roth, J. Phlorizin: a review. Diabetes Metab. Res. Rev. 21, 31–38 (2005).
Ellsworth, B. A. et al. Aglycone exploration of C-arylglucoside inhibitors of renal sodium-dependent glucose transporter SGLT2. Bioorg. Med. Chem. Lett. 18, 4770–4773 (2008).
Meng, W. et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 51, 1145–1149 (2008).
Nomura, S. et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J. Med. Chem. 53, 6355–6360 (2010).
Obermeier, M. et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab. Dispos. 38, 405–414 (2010).
Ferrannini, E., Ramos, S. J., Salsali, A., Tang, W. & List, J. F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33, 2217–2224 (2010).
Bailey, C. J., Gross, J. L., Pieters, A., Bastien, A. & List, J. F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomized, double-blind, placebo-controlled trial. Lancet 375, 2223–2233 (2010).
Wilding, J. P. et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 32, 1656–1662 (2009).
Kasichayanula, S. et al. Effect of a high-fat meal on the pharmacokinetics of dapagliflozin, a selective SGLT2 inhibitor, in healthy subjects. Diabetes Obes. Metab. 13, 770–773 (2011).
Kasichayanula, S. et al. Lack of pharmacokinetic interaction between dapagliflozin, a novel sodium-glucose transporter 2 inhibitor, and metformin, pioglitazone, glimepiride or sitagliptin in healthy subjects. Diabetes Obes. Metab. 13, 47–54 (2011).
Hussey, E. K. et al. Multiple-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy overweight and obese subjects: a randomized double-blind study. J. Clin. Pharmacol. 50, 636–646 (2010).
Fujimori, Y. et al. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J. Pharmacol. Exp. Ther. 327, 268–276 (2008).
Kashiwagi, A. et al. Ipragliflozin improved glycemic control with additional benefits of reductions of body weight and blood pressure in Japanese patients with type 2 diabetes mellitus: BRIGHTEN Study [abstract]. Diabetologia 54 (Suppl. 1), S68, a149 (2011).
Seman, L. et al. ENCORE: efficacy and safety of BI 10773, a new sodium glucose co-transporter-2 (SGLT-2) inhibitor, in type 2 diabetes patients inadequately controlled on metformin [abstract]. Diabetologia 54 (Suppl. 1), S67, a147 (2011).
Powell, D. et al. Single doses of LX4211, a dual inhibitor of SGLT1 and SGLT2, improves parameters of glycaemic control and increases GLP-1 and PYY in patients with type 2 diabetes [abstract]. Diabetologia 54 (Suppl. 1), S69, a150 (2011).
Isaji, M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int. Suppl. 120, S14–S19 (2011).
Yamamoto, K. et al. TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycemic activity. Br. J. Pharmacol. 164, 181–191 (2011).
Yao, C. H. et al. Discovery of novel N-β-D-xylosylindole derivatives as sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes. J. Med. Chem. 54, 166–178 (2011).
Kadokura, T. et al. The effect of renal impairment on the pharmacokinetics and urinary glucose excretion of the SGLT2 inhibitor ipragliflozin (ASP1941) in Japanese type 2 diabetes mellitus patients [abstract]. Diabetologia 54 (Suppl. 1), S346, a847 (2011).
Abdul-Ghani, M. A., Norton, L. & DeFronzo, R. A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 32, 515–531 (2011).
Piya, M. K., Tahrani, A. A. & Barnett, A. H. Emerging treatment options for type 2 diabetes. Br. J. Clin. Pharmacol. 70, 631–644 (2010).
Ghosh, R. K., Ghosh, S. M., Chawla, S. & Jasdanwala, S. A. SGLT2 inhibitors: a new emerging therapeutic class in the treatment of type 2 diabetes mellitus. J. Clin. Pharmacol. http://dx.doi.org/10.1177/0091270011400604
Komoroski, B. et al. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin. Pharmacol. Ther. 85, 513–519 (2009).
Osorio, H. et al. Effect of phlorizin on SGLT2 expression in the kidney of diabetic rats. J. Nephrol. 23, 541–546 (2010).
Vallon, V., Richter, K., Blantz, R. C., Thomson, S. & Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J. Am. Soc. Nephrol. 10, 2569–2576 (1999).
Hannedouche, T. P. et al. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int. 37, 1126–1133 (1990).
Vervoort, G., Veldman, B., Berden, J. H., Smits, P. & Wetzels, J. F. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur. J. Clin. Invest. 35, 330–336 (2005).
List, J. F., Woo, V., Morales, E., Tang, W. & Fiedorek, F. T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32, 650–657 (2009).
Quiñones Galvan, A. et al. Effect of insulin on uric acid excretion in humans. Am. J. Physiol. 268, E1–E5 (1995).
Doblado, M. & Moley, K. H. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am. J. Physiol. Endocrinol. Metab. 297, E831–E835 (2009).
Zhang, L., Feng, Y., List, J., Kasichayanula, S. & Pfister, M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes. Metab. 12, 510–516 (2010).
Lam, T. K. Neuronal regulation of homeostasis by nutrient sensing. Nat. Med. 16, 392–395 (2010).
Yu, A. S. et al. Functional expression of SGLTs in rat brain. Am. J. Physiol. Cell Physiol. 299, C1277–C1284 (2010).
FDA. FDA Briefing Document. NDA 202293. Dapagliflozin Tablets, 5 and 10 mg. Sponsor: Bristol-Myers Squibb. Advisory Committee Meeting, July 19, 2011 [online]. (2011).
Nauck, M. A. et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 34, 2015–2022 (2011).
Johnston, S. S. et al. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 34, 1164–1170 (2011).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article (researching data for the article, writing the manuscript, discussions of the content and review or editing of the manuscript before submission).
Corresponding author
Ethics declarations
Competing interests
E. Ferrannini declares associations with the following companies: Astellas (consultant), Bristol-Myers Squibb/AstraZeneca (consultant), Boehringer Ingelheim (consultant, grant/research support). See the article online for full details of the relationships. A. Solini declares no competing interests.
Rights and permissions
About this article
Cite this article
Ferrannini, E., Solini, A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 8, 495–502 (2012). https://doi.org/10.1038/nrendo.2011.243
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.243
This article is cited by
-
Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Cardiovascular Diabetology (2024)
-
SGLT2 Inhibitors in Aging-Related Cardiovascular Disease: A Review of Potential Mechanisms
American Journal of Cardiovascular Drugs (2023)
-
Real-world assessment of effectiveness and safety profile of remogliflozin etabonate in management of type 2 diabetes mellitus
International Journal of Diabetes in Developing Countries (2023)
-
Geriatrische Aspekte bei Diabetes mellitus (Update 2023)
Wiener klinische Wochenschrift (2023)
-
Comparisons of pleiotropic effects of SGLT2 inhibition and GLP-1 agonism on cardiac glucose intolerance in heart dysfunction
Molecular and Cellular Biochemistry (2022)