Abstract
The importance of sleep to hormones and glucose metabolism was first documented more than four decades ago. Since then, sleep curtailment has become an endemic behavior in modern society. In addition, the prevalence of sleep disorders, particularly obstructive sleep apnea (OSA), has increased. OSA is very common in endocrine and metabolic disorders, but often remains undiagnosed. This Review summarizes the laboratory and epidemiologic evidence that suggests how sleep loss, either behavioral or disease-related, and poor quality of sleep might promote the development of obesity and diabetes mellitus, and exacerbate existing endocrine conditions. Treatment of sleep disorders has the potential to improve glucose metabolism and energy balance. Screening for habitual sleep patterns and OSA might be critically important for patients with endocrine and metabolic disorders.
Key Points
-
Sleep loss, be it behavioral or related to sleep disorders, is an increasingly common condition in modern society
-
Experimental reduction of the duration or quality of sleep has a deleterious effect on glucose metabolism
-
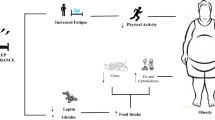
Experimental reduction of sleep duration downregulates the satiety hormone, leptin, upregulates the appetite-stimulating hormone, ghrelin, and increases hunger and appetite
-
Numerous cross-sectional and prospective, epidemiologic studies have provided evidence of an association between short-duration and/or poor-quality sleep and the prevalence or incidence of diabetes mellitus or obesity
-
Effective treatment of obstructive sleep apnea, a sleep disorder that is highly prevalent in metabolic and endocrine disorders, has the potential to improve glucose metabolism and energy balance
-
Screening for habitual sleep patterns and obstructive sleep apnea might be critically important for patients with endocrine and metabolic disorders
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gronfier, C. & Brandenberger, G. Ultradian rhythms in pituitary and adrenal hormones: their relations to sleep. Sleep Med. Rev. 2, 17–29 (1998).
Van Cauter, E. Endocrine physiology. In Principles and Practice of Sleep Medicine, 4th edn (eds Kryger, M., Roth, T. & Dement, W. C.) 266–282 (Elsevier–Saunders, Philadelphia, 2005).
Sakurai, T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 8, 171–181 (2007).
Adamantidis, A. & de Lecea, L. The hypocretins as sensors for metabolism and arousal. J. Physiol. 587, 33–40 (2009).
Wu, M. F., John, J., Maidment, N., Lam, H. A. & Siegel, J. M. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1079–R1086 (2002).
Estabrooke, I. V. et al. Fos expression in orexin neurons varies with behavioral state. J. Neurosci. 21, 1656–1662 (2001).
Zeitzer, J. M., Buckmaster, C. L., Lyons, D. M. & Mignot, E. Increasing length of wakefulness and modulation of hypocretin-1 in the wake-consolidated squirrel monkey. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1736–R1742 (2007).
National Sleep Foundation. 2008 “Sleep in America” poll, summary of findings 1–45 [online 19 Jan 2009] http://www.sleepfoundation.org/atf/cf/%7Bf6bf2668-a1b4-4fe8-8d1a-a5d39340d9cb%7D/2008%20POLL%20SOF.pdf (accessed 19 January 2009).
Institut National de Prévention et d'Education pour la Santé. Les français et leur sommeil. 1–12 [French] [online 19 Jan 2009] http://www.inpes.sante.fr/70000/dp/08/dp080310.pdf (accessed 19 January 2009).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Bergman, R. N. Minimal model: perspective from 2005. Hormone Res. 64 (Suppl. 3), S8–S15 (2005).
Spiegel, K., Knutson, K., Leproult, R., Tasali, E. & Van Cauter, E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J. Appl. Physiol. 99, 2008–2019 (2005).
Buxton, O. M., Pavlova, M. K., Reid, E., Simonson, D. C. & Adler, G. K. Sleep restriction for one week reduces insulin sensitivity measured using the eugylcemic hyperinsulinemic clamp technique. [online 12 June 2008] http://www.journalsleep.org/PDF/AbstractBook2008.pdf (accessed 27 January 2009).
Tasali, E., Leproult, R., Ehrmann, D. A. & Van Cauter, E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl Acad. Sci. USA 105, 1044–1049 (2008).
Bergman, R. N. Toward physiological understanding of glucose tolerance. Minimal model approach. Diabetes 38, 1512–1527 (1989).
Knutson, K. L. & Van Cauter, E. Associations between sleep loss and increased risk of obesity and diabetes. Ann. N.Y. Acad. Sci. 1129, 287–304 (2008).
Van Cauter, E. & Knutson, K. Sleep and the epidemic of obesity in children and adults. Eur. J. Endocrinol. 159 (Suppl. 1), S59–S66 (2008).
Tasali, E., Leproult, R. & Spiegel, K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog. Cardiovasc. Dis. (in press).
Spiegel, K., Tasali, E., Penev, P. & Van Cauter, E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 141, 846–850 (2004).
Nedeltcheva, A. et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 89, 126–133 (2008).
Spiegel, K. et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 89, 5762–5771 (2004).
Guilleminault, C. et al. Preliminary observations on the effects of sleep time in a sleep-restriction paradigm. Sleep Med. 4, 177–184 (2003).
Schmid, S. M., Hallschmid, M., Jauch-Chara, K., Born, J. & Schultes, B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J. Sleep Res. 17, 331–334 (2008).
Taheri, S., Lin, L., Austin, D., Young, T. & Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body-mass index. PLoS Med. 1, e62 (2004).
Chaput, J. P., Despres, J. P., Bouchard, C. & Tremblay, A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 15, 253–261 (2007).
Littman, A. J. et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int. J. Obes. (Lond.) 31, 466–475 (2007).
Cappuccio, F. P. et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 31, 619–626 (2008).
Chen, X., Beydoun, M. A. & Wang, Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 16, 265–274 (2008).
Patel, S. R. & Hu, F. B. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 16, 643–653 (2008).
Patel, S. R. et al. The association between sleep duration and obesity in older adults. Int. J. Obes (Lond.) 32, 1825–1834 (2008).
Berkey, C. S., Rockett, H. R. & Colditz, G. A. Weight gain in older adolescent females: the internet, sleep, coffee, and alcohol. J. Pediatr. 153, 635–639 (2008).
Keith, S. W. et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int. J. Obes. (Lond.) 30, 1585–1594 (2006).
Young, T. Increasing sleep duration for a healthier (and less obese?) population tomorrow. Sleep 31, 593–594 (2008).
Horne, J. Too weighty a link between short sleep and obesity? Sleep 31, 595–596 (2008).
Lauderdale, D. S. et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am. J. Epidemiol. 164, 5–16 (2006).
Vgontzas, A. N. et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int. J. Obes (Lond.) 32, 801–809 (2008).
Sanders, M. Sleep breathing disorders. In Principles and Practice of Sleep Medicine (eds Kryger, M., Roth, T. & Dement, W. C.) 969–1157 (W.B Saunders Company, Philadelphia, 2005).
West, S. D., Nicoll, D. J. & Stradling, J. R. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 61, 945–950 (2006).
Einhorn, D. et al. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr. Pract. 13, 355–362 (2007).
Foster, G. E. et al. Sleep apnea in obese adults with type 2 diabetes: baseline results from sleep AHEAD study. Sleep 28 (Suppl.), A204 (2005). http://www.journalsleep.org/pdf/Abstractbook2005.pdf (accessed 16 February 2009).
Tasali, E., Van Cauter, E. & Ehrmann, D. Polycystic-ovary syndrome and obstrcutive sleep apnea. Sleep Med. Clin. 3, 37–46 (2008).
Vgontzas, A. N. et al. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J. Clin. Endocrinol. Metab. 86, 517–520 (2001).
Bottini, P. & Tantucci, C. Sleep apnea syndrome in endocrine diseases. Respiration 70, 320–327 (2003).
Tasali, E., Mokhlesi, B. & Van Cauter, E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133, 496–506 (2008).
Tasali, E. & Ip, M. S. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc. Am. Thorac. Soc. 5, 207–217 (2008).
Seicean, S. et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 31, 1001–1006 (2008).
Punjabi, N. M. & Beamer, B. A. Alterations in glucose disposal in sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 179, 235–240 (2009).
Reichmuth, K. J., Austin, D., Skatrud, J. B. & Young, T. Association of sleep apnea and type II diabetes: a population-based study. Am. J. Respir. Crit. Care Med. 172, 1590–1595 (2005).
Dawson, A. et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J. Clin. Sleep Med. 4, 538–542 (2008).
Hassaballa, H. A., Tulaimat, A., Herdegen, J. J. & Mokhlesi, B. The effect of continuous, positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath 9, 176–180 (2005).
Babu, A. R., Herdegen, J., Fogelfeld, L., Shott, S. & Mazzone, T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch. Intern. Med. 165, 447–452 (2005).
Harsch, I. A. et al. The effect of continuous, positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration 71, 252–259 (2004).
Brooks, B. et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effects of continuous positive airway pressure treatment on insulin responsiveness. J. Clin. Endocrinol. Metab. 79, 1681–1685 (1994).
West, S. D., Nicoll, D. J., Wallace, T. M., Matthews, D. R. & Stradling, J. R. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 62, 969–974 (2007).
Harsch, I. A. et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 169, 156–162 (2004).
Schahin, S. P. et al. Long-term improvement of insulin sensitivity during CPAP therapy in the obstructive sleep-apnoea syndrome. Med. Sci. Monit. 14, CR117–CR121 (2008).
Lindberg, E., Berne, C., Elmasry, A., Hedner, J. & Janson, C. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 7, 553–560 (2006).
Dorkova, Z., Petrasova, D., Molcanyiova, A., Popovnakova, M. & Tkacova, R. Effects of CPAP on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 134, 686–692 (2008).
Saarelainen, S., Lahtela, J. & Kallonen, E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J. Sleep Res. 6, 146–147 (1997).
Ip, S., Lam, K., Ho, C., Tsang, K. & Lam, W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 118, 580–586 (2000).
Smurra, M. et al. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2, 207–213 (2001).
Coughlin, S. R., Mawdsley, L., Mugarza, J. A., Wilding, J. P. & Calverley, P. M. Cardiovascular and metabolic effects of CPAP in obese men with OSA. Eur. Respir. J. 29, 720–727 (2007).
Trenell, M. I. et al. Influence of constant, positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes. Metab. 9, 679–687 (2007).
Teramoto, S. et al. Cardiovascular and metabolic effects of CPAP in obese obstructive sleep apnoea patients. Eur. Respir. J. 31, 223–225 (2008).
Vgontzas, A. N. et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur. J. Clin. Invest. 38, 585–595 (2008).
Phillips, B. G. et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J. Hypertens. 17, 1297–1300 (1999).
Phillips, B. G., Kato, M., Narkiewicz, K., Choe, I. & Somers, V. K. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am. J. Physiol. Heart Circ. Physiol. 279, H234–H237 (2000).
Harsch, I. A. et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur. Respir. J. 22, 251–257 (2003).
Pillar, G. & Shehadeh, N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care 31 (Suppl. 2), S303–S309 (2008).
Chin, K. et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 100, 706–712 (1999).
Sanner, B. M., Kollhosser, P., Buechner, N., Zidek, W. & Tepel, M. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur. Respir. J. 23, 601–604 (2004).
Rubinsztajn, R., Kumor, M., Byskiniewicz, K. & Chazan, R. The influence of 3 weeks therapy with continuous positive airway pressure on serum leptin and homocysteine concentration in patients with obstructive sleep apnea syndrome [Polish]. Pneumonol. Alergol. Pol. 74, 63–67 (2006).
Drummond, M. et al. Autoadjusting-CPAP effect on serum leptin concentrations in obstructive sleep apnoea patients. BMC Pulm. Med. 8, 21 (2008).
Takahashi, K. et al. Acylated ghrelin level in patients with OSA before and after nasal CPAP treatment. Respirology 13, 810–816 (2008).
Loube, D. I., Loube, A. A. & Erman, M. K. Continuous positive airway pressure treatment results in weight loss in obese and overweight patients with obstructive sleep apnea. J. Am. Diet. Assoc. 97, 896–897 (1997).
Redenius, R., Murphy, C., O'Neill, E., Al-Hamwi, M. & Zallek, S. N. Does CPAP lead to change in BMI? J. Clin. Sleep Med. 4, 205–209 (2008).
Kajaste, S., Brander, P. E., Telakivi, T., Partinen, M. & Mustajoki, P. A cognitive-behavioral weight-reduction program in the treatment of obstructive sleep apnea syndrome, with or without initial nasal CPAP: a randomized study. Sleep Med. 5, 125–131 (2004).
Tasali, E. et al. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 3878–3884 (2008).
Subramanian, S., Desai, A., Joshipura, M. & Surani, S. Practice patterns of screening for sleep apnea in physicians treating PCOS patients. Sleep Breath 11, 233–237 (2007).
Netzer, N. C., Stoohs, R. A., Netzer, C. M., Clark, K. & Strohl, K. P. Using the Berlin Questionnaire to identify patients at risk for the sleep-apnea syndrome. Ann. Intern. Med. 131, 485–491 (1999).
Punjabi, N. M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 5, 136–143 (2008).
Young, T., Peppard, P. E. & Taheri, S. Excess weight and sleep-disordered breathing. J. Appl. Physiol. 99, 1592–1599 (2005).
Acknowledgements
Some research described in this article was supported by US National Institute of Health grants P01 AG-11412, R01 HL-075079, P60 DK-20595, R01 DK-0716960, R01 HL-075025 and M01 RR000055, by US Department of Defense award W81XWH-07-2-0071, by AASM/Pfizer Scholars Grant in Sleep Medicine (E Tasali), by Belgian 'Fonds de la Recherche Scientifique Médicale' (FRSM-3.4583.02), 'Fonds National de la Recherche Scientifique' (FNRS) and 'CARE Foundation' grants, by INSERM U628, and by Claude Bernard University of Lyon, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Eve Van Cauter has declared associations with the following companies: Actelyon (consultant) and Sanofi-Aventis (consultant). The other authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Spiegel, K., Tasali, E., Leproult, R. et al. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 5, 253–261 (2009). https://doi.org/10.1038/nrendo.2009.23
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.23
This article is cited by
-
The association between sleep duration and lung cancer: a meta-analysis
Sleep and Breathing (2024)
-
Is caregiver sleep quality an important clinical issue?
Sleep and Biological Rhythms (2024)
-
Sleep quality in relation to perceived psychological stress in patients with type 2 diabetes and in age- and sex-matched control individuals
Acta Diabetologica (2024)
-
Sleep characteristics of middle-aged adults with non-alcoholic fatty liver disease: findings from the Shahrekord PERSIAN cohort study
BMC Public Health (2023)
-
Lifestyle management in polycystic ovary syndrome – beyond diet and physical activity
BMC Endocrine Disorders (2023)