Abstract
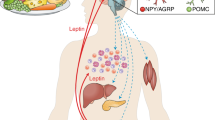
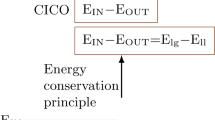
Childhood obesity has become epidemic over the past 30 years. The First Law of Thermodynamics is routinely interpreted to imply that weight gain is secondary to increased caloric intake and/or decreased energy expenditure, two behaviors that have been documented during this interval; nonetheless, lifestyle interventions are notoriously ineffective at promoting weight loss. Obesity is characterized by hyperinsulinemia. Although hyperinsulinemia is usually thought to be secondary to obesity, it can instead be primary, due to autonomic dysfunction. Obesity is also a state of leptin resistance, in which defective leptin signal transduction promotes excess energy intake, to maintain normal energy expenditure. Insulin and leptin share a common central signaling pathway, and it seems that insulin functions as an endogenous leptin antagonist. Suppressing insulin ameliorates leptin resistance, with ensuing reduction of caloric intake, increased spontaneous activity, and improved quality of life. Hyperinsulinemia also interferes with dopamine clearance in the ventral tegmental area and nucleus accumbens, promoting increased food reward. Accordingly, the First Law of Thermodynamics can be reinterpreted, such that the behaviors of increased caloric intake and decreased energy expenditure are secondary to obligate weight gain. This weight gain is driven by the hyperinsulinemic state, through three mechanisms: energy partitioning into adipose tissue; interference with leptin signal transduction; and interference with extinction of the hedonic response to food.
Key Points
-
Behavior has biochemical underpinnings, particularly the pathologic behaviors in disease states
-
Obesity is characterized by hyperinsulinemia and leptin resistance
-
In the long term, insulin functions as an endogenous leptin antagonist; it interferes with leptin signal transduction resulting in increased food intake and decreased physical activity
-
Chronic hyperinsulinemia interferes with satiety by preventing extinction of the hedonic response to food
-
Hyperinsulinemia has genetic, epigenetic, and environmental inputs
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ogden CL et al. (2002) Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288: 1728–1732
Hill JO et al. (2003) Obesity and the environment: where do we go from here? Science 299: 853–855
Ebbeling CB et al. (2002) Childhood obesity: public-health crisis, common sense cure. Lancet 360: 473–482
Troiano RP et al. (2000) Energy and fat intakes of children and adolescents in the United States: data from the National Health and Nutrition Examination Surveys. Am J Clin Nutr 72 (Suppl): 1343S–1353S
Kimm SYS et al. (2002) Decline in physical activity in black girls and white girls in adolescence. N Engl J Med 347: 709–715
Schwimmer JB et al. (2003) Health-related quality of life of severely obese children and adolescents. JAMA 289: 1813–1819
Epstein LH et al. (2001) Behavioral therapy in the treatment of pediatric obesity. Pediatr Clin North Am 48: 981–993
Ritchie L et al. (2001) Pediatric overweight: a review of the literature [http://www.cnr.berkeley.edu/cwh/PDFs/Full_COPI_secure.pdf] (accessed 17 May 2006)
Lustig RH (2001) The neuroendocrinology of childhood obesity. Pediatr Clin North Am 48: 909–930
Balthasar N et al. (2005) Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505
Lustig RH The efferent arm of the energy balance regulatory pathway: neuroendocrinology and pathology. In Obesity and Energy Metabolism: Research and Clinical Applications (Ed Donahoue PA) New Jersey: Humana, in press
Porte D et al. (2005) Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 54: 1264–1276
Flier JS (1998) What's in a name? In search of leptin's physiologic role. J Clin Endocrinol Metab 83: 1407–1413
Mark AL et al. (2003) A leptin-sympathetic-leptin feedback loop: potential implications for regulation of arterial pressure and body fat. Acta Physiol Scand 177: 345–349
Baskin DG et al. (1988) Insulin and insulin-like growth factors in the CNS. Trends Neurosci 11: 107–111
Schwartz MW et al. (1990) Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides 11: 467–472
Muntzel MS et al. (1994) Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol 267 (Pt 2): R1350–R1355
Niswender KD et al. (2001) Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413: 794–795
Brüning JC et al. (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125
Kubota N et al. (2004) Insulin receptor substrate 2 plays a crucial role in β cells and the hypothalamus. J Clin Invest 114: 917–927
Choudhury AI et al. (2005) The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest 115: 940–950
Niswender KD and Schwartz MW (2003) Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol 24: 1–10
Lowell BB and Spiegelman BM (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652–660
Collins S et al. (1996) Role of leptin in fat regulation [letter]. Nature 380: 677
Haynes WG et al. (1997) Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278
Blaak EE et al. (1993) Adrenoceptor subtypes mediating catecholamine-induced thermogenesis in man. Int J Obes Relat Metab Disord 17 (Suppl 3): S78–S81
Kreier F et al. (2002) Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-functional implications. J Clin Invest 110: 1243–1250
Lustig RH (2003) Autonomic dysfunction of the β-cell and the pathogenesis of obesity. Rev Endocr Metab Disord 4: 23–32
Yamada M et al. (2001) Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 410: 207–212
Bray GA and Greenway FL (1999) Current and potential drugs for treatment of obesity. Endocr Rev 20: 805–875
Leibel RL et al. (1995) Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628
Rosenbaum M et al. (1997) Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 82: 3647–3654
Rosenbaum M et al. (2003) Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192
Smit HJ et al. (2004) Mood and cognitive performance effects of “energy” drink constituents: caffeine, glucose, and carbonation. Nutr Neurosci 7: 127–139
Belza A and Jessen AB (2005) Bioactive food stimulants of sympathetic activity: effect on 24-h energy expenditure and fat oxidation. Eur J Clin Nutr 59: 733–741
Boden G et al. (1996) Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81: 454–458
Aronne LJ et al. (1995) Autonomic nervous system activity in weight gain and weight loss. Am J Physiol 269: R222–R225
Farooqi IS et al. (2002) Beneficial effects of leptin on obesity, T-cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110: 1093–1103
Lee HC et al. (1989) Direct effect of CNS on insulin hypersecretion in obese Zucker rats: involvement of vagus nerve. Am J Physiol 256: E439–E444
van Dijk G et al. (2005) Reduced anorexigenic efficacy of leptin, but not of the melanocortin receptor agonist melanotan-II, predicts diet-induced obesity in rats. Endocrinology 146: 5247–5256
Heymsfield SB et al. (1999) Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575
Poretti A et al. (2004) Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol 46: 220–229
Lustig RH et al. (2003) Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab 88: 611–616
Schofl C et al. (2002) Sympathoadrenal counterregulation in patients with hypothalamic craniopharyngioma. J Clin Endocrinol Metab 87: 624–629
Harz KJ et al. (2003) Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab 88: 5227–5231
Rosenbaum M et al. (2002) Low dose leptin administration reverses effects of sustained weight reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87: 2391–2394
Bray GA and Gallagher TF (1975) Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a review of the literature. Medicine (Baltimore) 54: 301–333
Lustig RH et al. (1999) Hypothalamic obesity in children caused by cranial insult: altered glucose and insulin dynamics, and reversal by a somatostatin agonist. J Pediatr 135: 162–168
Lustig RH et al. (2003) Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab 88: 2586–2592
Velasquez-Mieyer PA et al. (2003) Suppression of insulin secretion promotes weight loss and alters macronutrient preference in a subset of obese adults. Int J Obesity 27: 219–226
Lustig RH et al. (2004) Obesity, leptin resistance, and the effects of insulin suppression. Int J Obesity 28: 1344–1348
Lustig RH et al. (2006) A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes 30: 331–341
El-Haschimi K et al. (2000) Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832
Clegg DJ et al. (2005) Reduced anorexic effects of insulin in obesity-prone rats fed a moderate fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986
Mori H et al. (2004) Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743
Munzberg H and Myers MG (2005) Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8: 566–570
Emanuelli B et al. (2000) SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275: 15985–15991
Banks W et al. (1999) Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 20: 1341–1345
Ishihara Y et al. (2004) Effects of diet and time of the day on serum and CSF leptin levels in Osborne-Mendel and S5B/Pl rats. Obes Res 12: 1067–1076
Nam SY et al. (2001) Cerebrospinal fluid and plasma concentrations of leptin, NPY, and α-MSH in obese women and their relationship to negative energy balance. J Clin Endocrinol Metab 86: 4849–4853
Figlewicz DP et al. (1996) Review article: endocrine regulation of food intake and body weight. J Lab Clin Med 127: 328–332
Zabolotny JM et al. (2002) PTP1B regulates leptin signal transduction in vivo. Dev Cell 2: 489–495
Flier JS (2004) Obesity wars: molecular progress confronts an expanding epidemic. Cell 116: 337–350
Li HJ et al. (2005) A twin study for serum leptin, soluble leptin receptor, and free insulin-like growth factor-1 in pubertal females. J Clin Endocrinol Metab 90: 3659–3664
McLachlan KA et al. (2006) Do adiponectin, TNFα, leptin, and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diab Metab Res Rev 22: 131–138
Castracane VD et al. (2005) Serum leptin in nonpregnant and pregnant women and in old and new world nonhuman primates. Exp Biol Med 230: 251–254
Kelley AE et al. (2002) Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76: 365–377
Shalev U et al. (2001) Leptin attenuates food deprivation-induced relapse to heroin seeking. J Neurosci 21: RC129
Carr KD et al. (2003) Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience 119: 1157–1167
Wang GJ et al. (2001) Brain dopamine and obesity. Lancet 357: 354–357
Figlewicz DP et al. (1994) Intraventricular insulin increases dopaminergic transporter mRNA in rat VTA/substantia nigra. Brain Res 644: 331–334
Sipols AJ et al. (2002) Intraventricular insulin decreases kappa opioid-mediated sucrose intake in rats. Peptides 23: 2181–2187
Figlewicz DP (2003) Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Phyisol Regul Integ Comp Physiol 284: R882–R892
Arslanian SA et al. (2002) Hyperinsulinemia in African-American children. Decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51: 3014–3019
Preeyasombat C et al. (2005) Racial and etiopathologic dichotomies in insulin secretion and resistance in obese children. J Pediatr 146: 474–481
Stocker CJ et al. (2005) Fetal origins of insulin resistance and obesity. Proc Nutr Soc 64: 143–151
Yajnik CS et al. (2002) Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab 87: 5575–5580
Hofman PL et al. (2004) Premature birth and later insulin resistance. N Engl J Med 351: 2179–2186
Cettour-Rose P et al. (2005) Redistribution of glucose from skeletal muscle to adipose tissue during catch-up fat: a link between catch-up growth and later metabolic syndrome. Diabetes 54: 751–756
Isganaitis E and Lustig RH (2005) Fast food, central nervous system insulin resistance, and obesity. Arterioscler Thromb Vasc Biol 25: 2451–2462
Acknowledgements
The author would like to thank Drs WL Miller, MM Grumbach, SH Mellon, MF Dallman, ES Epel, AK Garber, JA Yanovski, and JG Kral for their input to and critique of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Lustig, R. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nat Rev Endocrinol 2, 447–458 (2006). https://doi.org/10.1038/ncpendmet0220
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0220
This article is cited by
-
Position statement on nutrition therapy for overweight and obesity: nutrition department of the Brazilian association for the study of obesity and metabolic syndrome (ABESO—2022)
Diabetology & Metabolic Syndrome (2023)
-
An integrated model of obesity pathogenesis that revisits causal direction
Nature Reviews Endocrinology (2022)
-
Competing paradigms of obesity pathogenesis: energy balance versus carbohydrate-insulin models
European Journal of Clinical Nutrition (2022)
-
Environmental lipidomics: understanding the response of organisms and ecosystems to a changing world
Metabolomics (2020)
-
Inflammation Markers in Type 2 Diabetes and the Metabolic Syndrome in the Pediatric Population
Current Diabetes Reports (2018)