Abstract
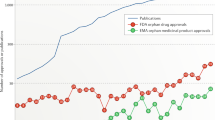
The 1983 US Orphan Drug Act has stimulated the development of new therapies for rare diseases. To provide the first comprehensive overview of orphan-designated products and their indications, this article quantitatively analyses the characteristics and distribution of orphan designations and approvals by the US Food and Drug Administration from 1983 to August 2008. Of the 1,892 orphan-designated products, 326 received marketing approval, representing 247 different drugs and more than 200 different diseases. About half of the approvals had occurred by 4 years after designation was granted. The most common patient population size for orphan designations and approvals was fewer than 10,000 patients, and cancer was the most common disease area. The implications of such findings for future development and marketing of therapies for rare diseases are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Orphan Drug Act, H.R. 5238, Public Law No. 97–414, 97th Congress (1983).
US Food and Drug Administration. Developing products for rare diseases & conditions. US FDA website [online], (2010).
Shieppati, A., Henter, J., Daina, E. & Aperia A. Why rare diseases are an important medical and social issue. Lancet 371, 2039–2041 (2008).
Remuzzi, G. & Garattini, S. Rare diseases: what's next? Lancet 371, 1978–1979 (2008).
Haffner, M. E. Adopting orphan drugs — two dozen years of treating rare diseases. N. Engl. J. Med. 354, 445–447 (2006).
The European Parliament and the Council of the European Union. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. Official J. Eur. Communities L 18/1–L 18/5 (2000).
National Institutes of Health, Office of Rare Diseases. Rare diseases and related terms. NIH website [online], (2009).
Johns Hopkins Bloomberg School of Public Health. Fundamentals of Epidemiology. Life Tables. Johns Hopkins Bloomberg School of Public Health website [online], (2008).
Acknowledgements
We thank G. Gupta for statistical consultation, J. Fritsch and H. Startzman for providing useful background on orphan designations at the FDA, K. Robertson for provision of FDA data abstracts and A. Kelkar for the data and analysis on NMEs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
At the time the work on this project was performed, all authors were FDA employees or fellows. Subsequently, M. Miles Braun was employed by MedImmune, LLC and Sanofi Pasteur.
Supplementary information
Supplementary box 1
Statistical analysis (PDF 190 kb)
Rights and permissions
About this article
Cite this article
Braun, M., Farag-El-Massah, S., Xu, K. et al. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov 9, 519–522 (2010). https://doi.org/10.1038/nrd3160
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3160
This article is cited by
-
A clinical study and future prospects for bioactive compounds and semi-synthetic molecules in the therapies for Huntington's disease
Molecular Neurobiology (2024)
-
Achieving big with small: quantitative clinical pharmacology tools for drug development in pediatric rare diseases
Journal of Pharmacokinetics and Pharmacodynamics (2023)
-
Protocol paper: a multi-center, double-blinded, randomized, 6-month, placebo-controlled study followed by 12-month open label extension to evaluate the safety and efficacy of Saracatinib in Fibrodysplasia Ossificans Progressiva (STOPFOP)
BMC Musculoskeletal Disorders (2022)
-
Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases
Orphanet Journal of Rare Diseases (2021)
-
DDIEM: drug database for inborn errors of metabolism
Orphanet Journal of Rare Diseases (2020)