Key Points
-
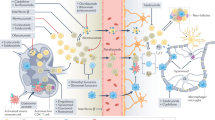
Lymphocytes flowing in the blood vessels are induced to slow, roll along the blood vessel walls and finally arrest their movement and attach to the endothelium through a complex sequence of events. Investigators had long searched for molecules that would allow the specific attachment of lymphocytes to the site of inflammation. Immunologists had proposed that specific molecular 'zip codes' were crucial for the entry of lymphocytes to specific tissues. A different zip code might exist for brain, synovium and gut, for example.
-
α4β1integrin is key for binding of lymphocytes to the walls of blood vessels in the inflamed brain.
-
α4β1integrin is also crucial for entry to the β-cells in the pancreas, the gut and the synovium, rendering the zip-code hypothesis problematic.
-
Preclinical development of a drug to block lymphocyte homing was focused on the animal model of multiple sclerosis, known as experimental autoimmune encephalomyelitis (EAE). In ongoing EAE, where disease has already been established with evidence of clinical paralysis of the hind limbs, anti-α4β1 integrin antibodies reversed the paralysis and cleared the brain of ongoing lymphocytic infiltration
-
A humanized monoclonal antibody to α4β1integrin, known as natalizumab, was taken into the clinic for patients with relapsing remitting multiple sclerosis. Phase II clinical trial results with natalizumab showed a reduction in lesions on magnetic resonace imaging and a reduction in the relapse rate in patients with multiple sclerosis.
-
Phase III clinical trials demonstrated a 66% reduction in the rate of relapse after 1 year, leading to approval of the drug for treatment of relapsing remitting multiple sclerosis. Promising clinical trial results in Phase II were reported for Crohn's disease and rheumatoid arthritis as well.
-
Three months after the FDA approval the drug was voluntarily withdrawn from the market, after two cases of the deadly disease progressive multifocal leukoencephalopathy (PML) were reported in patients who took the drug in clinical trials for multiple sclerosis. A third patient in a trial for Crohn's disease died of PML. At present natalizumab is off the market for all its indications, and whether it will return to the market is uncertain.
Abstract
Immunologists have long hypothesized that particular 'molecular addresses' govern lymphocyte entry to a given organ. In 1992, α4β1 integrin was identified as the key molecule involved in homing to inflamed regions of the brain. An antibody to α4β1integrin blocked paralysis in an animal model of multiple sclerosis, and the humanized monoclonal antibody natalizumab, which binds α4β1 integrin, reduced relapses 66% in clinical trials in multiple sclerosis. Three months after its expedited approval by the FDA, natalizumab was removed from the market after two cases of deadly progressive multifocal leukoencephalopathy were reported among the few thousand patients who had taken this drug in those clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Steinman, L. Multiple sclerosis: a two-stage disease. Nature Immunol. 2, 762–5 (2001).
Kiesierer, B. C. & Hartung, H. P. Current disease modifying therapies in mutlple sclerosis. Semin. Neurol. 23, 133–146 (2003).
Zamvil, S. & Steinman, L. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron 38, 685–688 (2003).
Steinman, L. & Zamvil, S. Transcriptional analysis of targets in multiple sclerosis. Nature Rev. Immunol. 3, 483–493 (2003).
Platten, M. & Steinman, L. Multiple sclerosis: trapped in deadly glue. Nature Med. 11, 252–253 (2005).
Yednock, T. et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature 356, 63–66 (1992). The first description of the crucial role of α4β1 integrin in the homing of lymphocytes to inflamed brain. Antibodies to α4β1 integrin blocked paralysis in EAE.
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994). Excellent early review of the physiology of lymphocyte adhesion to inflamed endothelium.
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996). Another excellent review of the physiology of lymphocyte adhesion to inflamed endothelium.
von Adrian, U. H. & Mackay, C. R. T cell function and migration: two sides of the same coin. N. Engl. J. Med. 343, 1020–1024 (2000).
Steinman, L., Rosenbaum, J. T., Sriram, S. & McDevitt, H. O. In vivo effects of antibodies to immune response gene products: prevention of experimental allergic encephalitis. Proc. Natl Acad. Sci. USA 78, 7111–7114 (1981).
Sakai, K., Tabira, T., Endoh, M. & Steinman, L. Ia expression in chronic relapsing experimental allergic encephalomyelitis induced by long-term cultured T cell lines in mice. Lab. Invest. 54, 345–352 (1986).
Steinman, L., Solomon, D., Zamvil, S., Lim, M. & Sriram, S. Prevention of EAE with anti I-A antibody: Decreased accumulation of radiolabeled lymphocytes in the central nervous system. J. Neuroimmunol. 5, 91–97 (1983).
Sriram, S. & Steinman, L. Anti I-A antibody suppresses active encephalomyelitis: treatment model for IR gene linked diseases. J. Exp. Med. 158, 1362–1367 (1983).
Friedmann, A., Frankel, G., Lorch, Y. & Steinman, L. Monoclonal anti I-A reverses chronic paralysis and demyelination in Theiler's virus infected mice: critical importance of timing of treatment. J. Virol. 61, 898–903 (1987).
Ben-Nun, A., Wekerle, H. & Cohen, I. R. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature 292, 60–62 (1981).
Zamvil, S. et al. T cell clones specific for myelin basic protein induce chronic relapsing EAE and demyelination. Nature 317, 355–358 (1985).
Stamper, H. B. & Woodruff, J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J. Exp. Med. 144, 828–833 (1976).
Brocke, S., Piercy, C., Steinman, L., Weissman, I. L. & Veromaa, T. Antibodies to CD44 and integrin α4, but not L-selectin, prevent CNS inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc. Natl Acad. Sci. USA 96, 6896–6901 (1999).
Baron, J. L., Madri, J. A., Ruddle, N. H., Hashim, G. & Janeway, C. A. Jr. Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 177, 57–68 (1993).
Verbeek, M. M., Westphal, J. R., Ruiter, D. J. & de Waal, R. M. T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J. Immunol. 154, 5876–5884 (1995).
Kent, S. J. et al. A monoclonal antibody to α4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J. Neuroimmunol. 58, 1–10 (1995).
Keszthelyi, E. et al. Evidence for a prolonged role of α4 integrin throughout active experimental allergic encephalomyelitis. Neurology 47, 1053–1059 (1996).
Yang, X., Karin, N., Tisch, R., Steinman, L. & McDevitt, H. O. Inhibition of insulitis and prevention of diabetes in NOD mice by blocking L-selectin and VLA-4 adhesion receptors. Proc. Natl Acad. Sci. USA 90, 10494–10498 (1993).
Yang, X. et al. A predominant role of α4-integrin in the spontaneous development of autoimmune diabetes in NOD Mice. Proc. Natl Acad. Sci. USA 91, 12604–12608 (1994). Demonstrated that α4-integrin is important in homing to inflamed pancreas in a model of type 1 diabetes.
Rubin, S. A., Yednock, T. A. & Carbone, K. M. In vivo treatment with anti-α4 integrin suppresses clinical and pathological evidence of Borna disease virus infection. J. Neuroimmunol. 84, 158–163 (1998).
Sheremata, W. A., Vollmer, T. L., Stone, L. A., Willmer-Hulme, A. J. & Koller, M. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology 52, 1072–1074 (1999).
Theien, B. E. et al. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. J. Clin. Invest. 107, 995–1006 (2001). Worsening of EAE is described after administration of α4-integrin antibody.
Leger, J. P. et al. Humanisation of a mouse antibody against human α-4 integrin: a potential therapeutic for the treatment of multiple sclerosis. Hum. Antibodies 8, 3–16 (1997).
Tubridy, N. et al. The effect of anti-a4 integrin antibody on brain lesion activity in MS. Neurology 53, 466–472 (1999).
Miller, D. A. et al. A controlled trial of natalizumab for relapsing-remitting multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003). Phase II trial results show reduction in clinical relapses during a 6-month course of natalizumab.
US FDA. Natalizumab (marketed as Tysabri) Information [online], <http://www.fda.gov/cder/drug/infopage/natalizumab/default.htm> (2005). FDA website with the results of the Phase III clinical trials leading to the approval of natalizumab. This page also contains the labelling of the approved drug, and the notice of the voluntary withdrawal of the drug.
Caruso, M., Belloni, L., Sthandier, O., Amati, P. & Garcia, M. α4β1 integrin acts as a cell receptor for murine polyomavirus at the postattachment level. J. Virol. 77, 3913–3921 (2003).
Acknowledgements
I would like to thank K. Gijbels for the figure in Box 1.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
L.W. founded Neurocrine Biosciences and Bayhill Therapeutics. These companies are testing new therapies for multiple sclerosis. L.W. is a consultant to Neurocrine Biosciences and Bayhill Therapeutics.
Related links
Related links
DATABASES
Entrez Gene
OMIM
Glossary
- NATALIZUMAB (TYSABRI)
-
The generic and trade name, respectively, for the humanized antibody to α4β1 integrin, approved in November 2004 for the treatment of multiple sclerosis.
- INTEGRIN
-
Proteins involved with the cytoskeleton that have extracellular domains comprising elements of α- and β-chains. The α4β1 integrin is involved in lymphocyte homing to the brain and to other organs.
- MULTIPLE SCLEROSIS
-
A demyelinating autoimmune disease of the brain and spinal cord affecting 400,000 individuals in the United States.
- EXPERIMENTAL AUTOIMMUNE ENCEPHALOMYELITIS (EAE)
-
An animal model of multiple sclerosis that was discovered 75 years ago.
- LYMPHOCYTE HOMING
-
Lymphocytes are mobile elements of the immune system that have the capacity to home from lymph nodes via the blood circulation to particular target sites.
Rights and permissions
About this article
Cite this article
Steinman, L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nat Rev Drug Discov 4, 510–518 (2005). https://doi.org/10.1038/nrd1752
Issue Date:
DOI: https://doi.org/10.1038/nrd1752