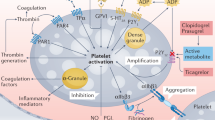
Abstract
Despite considerable reductions in cardiovascular events in patients with an acute coronary syndrome (ACS) receiving dual antiplatelet therapy (DAPT), substantial residual risk persists. This unmet need has stimulated the development of anticoagulant drugs that target specific coagulation factors involved in the pathogenesis of thrombosis after atheromatous plaque disruption. Factor Xa is an attractive target for inhibition because of both its integral role in coagulation and its recognized participation in cellular proliferation and inflammation. Several oral, direct factor Xa inhibitors are undergoing investigation and large, phase III clinical trials of two agents, apixaban and rivaroxaban, in patients with an ACS have been completed. On the basis of the known pathobiology of ACS, one might anticipate that drugs in this class of anticoagulant would beneficially reduce ischemic and thrombotic events; however, a strategy of combined anticoagulant therapy and DAPT is likely to increase concomitant bleeding complications. The balance of benefit and risk will ultimately determine uptake in clinical practice. We review the available data on factor Xa inhibitors in the long-term management of patients with an ACS.
Key Points
-
Factor Xa is an attractive target for inhibition in patients with an acute coronary syndrome (ACS) because of its roles in coagulation, cellular proliferation, apoptosis, matrix-metalloprotein stability, and inflammation
-
Four oral, competitive factor Xa inhibitors (apixaban, darexaban, letaxaban, and rivaroxaban) have been investigated in patients with an ACS; only apixaban and rivaroxaban have progressed to phase III clinical trials
-
In the APPRAISE-2 trial, addition of apixaban to standard therapy in patients with an ACS showed no clinical benefit, and increased the risk of major and intracranial bleeding
-
In ATLAS ACS 2–TIMI 51, addition of rivaroxaban to dual antiplatelet therapy (aspirin plus clopidogrel) reduced cardiovascular events and mortality, which outweighed the increased bleeding risk
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
Wiviott, S. D. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
Rothberg, M. B., Celestin, C., Fiore, L. D., Lawler, E. & Cook, J. R. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann. Intern. Med. 143, 241–250 (2005).
Bertrand, M. E. et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (FANTASTIC) study. Circulation 98, 1597–1603 (1998).
Hurlen, M., Abdelnoor, M., Smith, P., Erikssen, J. & Arnesen, H. Warfarin, aspirin, or both after myocardial infarction. N. Engl. J. Med. 347, 969–974 (2002).
Khurram, Z. et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J. Inv. Cardiol. 18, 162–164 (2006).
Rossini, R. et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am. J. Cardiol. 102, 1618–1623 (2008).
Anand, S. S. & Yusuf, S. Oral anticoagulants in patients with coronary artery disease. J. Am. Coll. Cardiol. 41 (Suppl. S), 62S–69S (2003).
Hoffman, M. & Monroe, D. M. 3rd. A cell-based model of hemostasis. Thromb. Haemost. 85, 958–965 (2001).
Herbert, J. et al. Effector protease receptor 1 mediates the mitogenic activity of factor Xa for vascular smooth muscle cells in vitro and in vivo. J. Clin. Invest. 101, 993–1000 (1998).
Versteeg, H. H., Spek, C. A., Richel, D. J. & Peppelenbosch, M. P. Coagulation factors VIIa and Xa inhibit apoptosis and anoikis. Oncogene 23, 410–417 (2004).
Rauch, B. H., Bretschneider, E., Braun, M. & Schror, K. Factor Xa releases matrix metalloproteinase-2 (MMP-2) from human vascular smooth muscle cells and stimulates the conversion of pro-MMP-2 to MMP-2: role of MMP-2 in factor Xa-induced DNA synthesis and matrix invasion. Circ. Res. 90, 1122–1127 (2002).
Cirino, G. et al. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J. Clin. Invest. 99, 2446–2451 (1997).
Eriksson, B. I., Quinlan, D. J. & Weitz, J. I. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin. Pharmacokinet. 48, 1–22 (2009).
Eriksson, B. I. et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N. Engl. J. Med. 358, 2765–2775 (2008).
Lassen, M. R. et al. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J. Thromb. Haemost. 5, 2368–2375 (2007).
Lassen, M. R. et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N. Engl. J. Med. 358, 2776–2786 (2008).
Lassen, M. R. et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N. Engl. J. Med. 363, 2487–2498 (2010).
Connolly, S. J. et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 364, 806–817 (2011).
Granger, C. B. et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 365, 981–992 (2011).
Patel, M. R. et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 365, 883–891 (2011).
Cabral, K. P. & Ansell, J. Oral direct factor Xa inhibitors for stroke prevention in atrial fibrillation. Nat. Rev. Cardiol. http://dx.doi.org/10.1038/nrcardio.2012.19
Furie, B. & Furie, B. C. Mechanisms of thrombus formation. N. Engl. J. Med. 359, 938–949 (2008).
Morrissey, J. H., Macik, B. G., Neuenschwander, P. F. & Comp, P. C. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood 81, 734–744 (1993).
Monroe, D. M., Hoffman, M. & Roberts, H. R. Transmission of a procoagulant signal from tissue factor-bearing cell to platelets. Blood Coagul. Fibrinolysis 7, 459–464 (1996).
Diaz-Ricart, M. et al. Thrombin facilitates primary platelet adhesion onto vascular surfaces in the absence of plasma adhesive proteins: studies under flow conditions. Haematologica 85, 280–288 (2000).
Monkovic, D. D. & Tracy, P. B. Functional characterization of human platelet-released factor V and its activation by factor Xa and thrombin. J. Biol. Chem. 265, 17132–17140 (1990).
Monkovic, D. D. & Tracy, P. B. Activation of human factor V by factor Xa and thrombin. Biochemistry 29, 1118–1128 (1990).
Hultin, M. B. Modulation of thrombin-mediated activation of factor VIII:C by calcium ions, phospholipid, and platelets. Blood 66, 53–58 (1985).
Bono, F. et al. Human umbilical vein endothelial cells express high affinity receptors for factor Xa. J. Cell. Physiol. 172, 36–43 (1997).
Pejler, G., Lunderius, C. & Tomasini-Johansson, B. Macrophages synthesize factor X and secrete factor X/Xa-containing prothrombinase activity into the surrounding medium. Thromb. Haemost. 84, 429–435 (2000).
Altieri, D. C. & Edgington, T. S. Identification of effector cell protease receptor-1. A leukocyte-distributed receptor for the serine protease factor Xa. J. Immunol. 145, 246–253 (1990).
Macfarlane, R. G. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature 202, 498–499 (1964).
Yin, E. T. & Wessler, S. Investigation of the apparent thrombogenicity of thrombin. Thromb. Diath. Haemorrh. 20, 465–468 (1968).
Ieko, M. et al. Synthetic selective inhibitors of coagulation factor Xa strongly inhibit thrombin generation without affecting initial thrombin forming time necessary for platelet activation in hemostasis. J. Thromb. Haemost. 2, 612–618 (2004).
Petros, S., Siegemund, T., Siegemund, A. & Engelmann, L. The effect of different anticoagulants on thrombin generation. Blood Coagul. Fibrinolysis 17, 131–137 (2006).
Furugohri, T., Sugiyama, N., Morishima, Y. & Shibano, T. Antithrombin-independent thrombin inhibitors, but not direct factor Xa inhibitors, enhance thrombin generation in plasma through inhibition of thrombin–thrombomodulin–protein C system. Thromb. Haemost. 106, 1076–1083 (2011).
Morishima, Y., Furugohri, T., Shiozaki, Y., Sugiyama, N. & Shibano, T. Antithrombin-independent thrombin inhibitors, but not factor Xa inhibitors, enhance thrombin generation in human plasma via inhibition of thrombin-thrombomodulin–protein C system [abstract]. Blood 108, a914 (2006).
Food and Drug Administration, Cardiovascular and Renal Drugs Advisory Committee. Briefing information [online] (2004).
Ansell, J. Factor Xa or thrombin: is factor Xa a better target? J. Thromb. Haemost. 5 (Suppl. 1), 60–64 (2007).
Yusuf, S. et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N. Engl. J. Med. 354, 1464–1476 (2006).
Yusuf, S. et al. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA 295, 1519–1530 (2006).
Alexander, J. H. et al. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation 119, 2877–2885 (2009).
Turpie, A. G. et al. A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT). Thromb. Haemost. 101, 68–76 (2009).
Steg, P. G. et al. RUBY-1: a randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM150) following acute coronary syndrome. Eur. Heart J. 32, 2541–2554 (2011).
Ogata, K. et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J. Clin. Pharmacol. 50, 743–753 (2010).
Kohrt, J. T. et al. The discovery of (2R,4R)-N-(4-chlorophenyl)-N-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)-4-methoxypyrrolidine-1,2-dicarboxamide (PD 0348292), an orally efficacious factor Xa inhibitor. Chem. Biol. Drug Des. 70, 100–112 (2007).
Goldstein, S. et al. Safety evaluation of the factor Xa inhibitor TAK-442 in subjects with acute coronary syndromes: phase 2 AXIOM-ACS trial results [abstract]. Eur. Heart J. 32 (Abstract Suppl.), 414 (2011).
Agnelli, G. et al. A phase II study of the oral factor Xa inhibitor LY517717 for the prevention of venous thromboembolism after hip or knee replacement. J. Thromb. Haemost. 5, 746–753 (2007).
Mega, J. L. et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS–TIMI 46): a randomised, double-blind, phase II trial. Lancet 374, 29–38 (2009).
Pinto, D. J. et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J. Med. Chem. 50, 5339–5356 (2007).
Raghavan, N. et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab. Dispos. 37, 74–81 (2009).
Karthikeyan, G. & Eikelboom, J. W. Apixaban in acute coronary syndromes. Cardiovasc. Ther. 29, 285–290 (2011).
Wang, L. et al. In vitro assessment of metabolic drug–drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab. Dispos. 38, 448–458 (2010).
Schulman, S. & Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 3, 692–694 (2005).
Alexander, J. H. et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N. Engl. J. Med. 365, 699–708 (2011).
Chesebro, J. H. et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 76, 142–154 (1987).
Davis, E. M., Packard, K. A., Knezevich, J. T. & Campbell, J. A. New and emerging anticoagulant therapy for atrial fibrillation and acute coronary syndrome. Pharmacotherapy 31, 975–1016 (2011).
Weinz, C., Radtke, M., Schmeer, K., Kern, A. & Pleiss, U. In vitro metabolism of BAY 59-7939—an oral, direct factor Xa inhibitor. Drug Metab. Rev. 36, 98–98 (2004).
Weinz, C. et al. Metabolism and distribution of [C14]BAY 59-7939—an oral, direct factor Xa inhibitor—in rat, dog and human. Drug Metab. Rev. 36, 98–98 (2004).
Mega, J. L. et al. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 366, 9–19 (2012).
Mehta, R. S. Novel oral anticoagulants for prophylaxis and treatment of venous thromboembolism: part I (factor Xa inhibitors). Expert Rev. Hematol. 3, 227–241 (2010).
Astellas Pharma. Astellas Pharma Inc. discontinues development of darexaban (YM150), an oral direct factor Xa inhibitor[online], (2011).
Fujimoto, T. et al. Discovery of a tetrahydropyrimidin-2(1H)-one derivative (TAK-442) as a potent, selective, and orally active factor Xa inhibitor. J. Med. Chem. 53, 3517–3531 (2010).
Weitz, J. I. New oral anticoagulants in development. Thromb. Haemost. 103, 62–70 (2010).
Weitz, J. I. et al. A dose-finding study with TAK-442, an oral factor Xa inhibitor, in patients undergoing elective total knee replacement surgery. Thromb. Haemost. 104, 1150–1157 (2010).
Lee, C. W. et al. Comparison of ruptured coronary plaques in patients with unstable and stable clinical presentation. J. Thromb. Thrombolysis 32, 150–157 (2011).
Undas, A., Brummel, K., Musial, J., Mann, K. G. & Szczeklik, A. Blood coagulation at the site of microvascular injury: effects of low-dose aspirin. Blood 98, 2423–2431 (2001).
Brummel, K. E., Paradis, S. G., Butenas, S. & Mann, K. G. Thrombin functions during tissue factor-induced blood coagulation. Blood 100, 148–152 (2002).
Mann, K. G. Thrombin generation in hemorrhage control and vascular occlusion. Circulation 124, 225–235 (2011).
Haynes, L. M., Dubief, Y. C., Orfeo, T. & Mann, K. G. Dilutional control of prothrombin activation at physiologically relevant shear rates. Biophys. J. 100, 765–773 (2011).
Diener, H. C. et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 364, 331–337 (2004).
Bauersachs, R. et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 363, 2499–2510 (2010).
Kubitza, D., Becka, M., Voith, B., Zuehlsdorf, M. & Wensing, G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin. Pharmacol. Ther. 78, 412–421 (2005).
Roe, M. T. & Ohman, E. M. A new era in secondary prevention after acute coronary syndrome. N. Engl. J. Med. 366, 85–87 (2011).
Tricoci, P. et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N. Engl. J. Med. 366, 20–33 (2011).
Lu, G. et al. Recombinant antidote for reversal of anticoagulation by factor Xa inhibitors [abstract]. Blood 112, a983 (2008).
Turpie, A. G. Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 27, 1238–1247 (2007).
Iwatsuki, Y. et al. Biochemical and pharmacological profiles of YM150, an oral direct factor Xa inhibitor [abstract]. Blood 108, a273 (2006).
Groenendaal-van de Meent, D. et al. YM150, an oral direct inhibitor of factor Xa, demonstrated a predictable and dose-proportional pharmacokinetic/pharmacodynamic profile after single and multiple dosing: results from three studies [abstract]. Blood 116, a3323 (2010).
Saiah, E. & Soares, C. Small molecule coagulation cascade inhibitors in the clinic. Curr. Top. Med. Chem. 5, 1677–1695 (2005).
Perzborn, E. et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J. Thromb. Haemost. 3, 514–521 (2005).
Lu, G. et al. Reversal of rivaroxaban mediated anticoagulation in animal models by a recombinant antidote protein (r-Antidote, PRT064445) [abstract]. Eur. Heart J. 32 (Abstract Suppl.), 640–641 (2011).
Kawamura, M. et al. Antithrombotic and anticoagulant profiles of TAK-442, a novel factor Xa inhibitor, in a rabbit model of venous thrombosis. J. Cardiovasc. Pharmacol. 56, 156–161 (2010).
Nakamura, K. et al. Comparison of the pharmacokinetics and pharmacodynamics of TAK-442, a novel factor Xa inhibitor, between Japanese acute coronary syndrome patients and healthy Japanese males. J. Thromb. Haemost. 9, 107–107 (2011).
Gibson, C. M. et al. Rationale and design of the Anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome—thrombolysis in myocardial infarction 51 (ATLAS ACS 2–TIMI 51) trial: a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of rivaroxaban in subjects with acute coronary syndrome. Am. Heart J. 161, 815–821e6 (2011).
Author information
Authors and Affiliations
Contributions
J. W. Wisler researched the data for this article. Both authors contributed substantially to discussion of its contents and to writing, reviewing, and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R. C. Becker has received grants or research support from the following companies: AstraZeneca, Bayer Pharmaceuticals, Bristol–Myers Squibb, Daiichi Sankyo, Johnson & Johnson, The Medicines Company, Momenta, Regado Biosciences, and Schering–Plough. Additionally, he is, or has been, a consultant for Eli Lilly and Merck. J. W. Wisler declares no competing interests.
Rights and permissions
About this article
Cite this article
Wisler, J., Becker, R. Oral factor Xa inhibitors for the long-term management of ACS. Nat Rev Cardiol 9, 392–401 (2012). https://doi.org/10.1038/nrcardio.2012.18
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2012.18
This article is cited by
-
Antithrombotic therapy for patients with STEMI undergoing primary PCI
Nature Reviews Cardiology (2017)
-
Novel antiplatelet agents in acute coronary syndrome
Nature Reviews Cardiology (2015)
-
Oral direct factor Xa inhibitors for stroke prevention in atrial fibrillation
Nature Reviews Cardiology (2012)