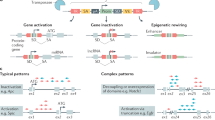
Key Points
-
The integration of slow transforming retroviruses into the genome of host cells can lead to deregulation of nearby genes. Cells can be infected repeatedly, acquiring multiple mutations that can lead to the development of a tumour.
-
Genes implicated in tumour formation are found through the identification of proviral or transposon insertions in independent tumours at frequencies higher than would be expected by chance. Genes in loci that recurrently contain proviral or transposon insertions in independent tumours show a significant overlap with known human oncogenes and tumour suppressor genes.
-
The arrival of next-generation sequencing technologies allows identification of most if not all insertions in a tumour. This will enable a more comprehensive identification of cancer genes and cooperating pairs of cancer genes and facilitate the establishment of the functional role of these cancer genes in tumour development.
-
Examining the genes that collaborate with common mutations in the Myc, p53, Rb and Ras pathways may identify genes that are essential for activating or abrogating the tumorigenic effects of these mutations. Using retrovirus- or transposon-mediated insertional mutagenesis to identify genes that collaborate with genes of the Myc, p53, Rb and Ras pathways may expand the range of targets for developing novel cancer therapeutics.
-
Retrovirus- or transposon-mediated insertional mutagenesis screens can be used to identify genes that confer resistance to drugs both in vitro and in vivo and may thus provide pivotal information to improve existing therapeutic strategies.
-
Although most proviral insertions are activating rather than inactivating, a substantial fraction of the insertions are found to abrogate gene function, thus enabling the identification of tumour suppressor genes.
-
Cross-comparison of insertional mutagenesis data from mice with mutation data from human tumour panels may help identify the 'driver' mutations in human cancer. With the development of insertional mutagenesis strategies for solid tissues these cross-comparisons will become even more powerful, as they will allow direct comparison of human tumour data with insertions derived from their cognate mouse tumours.
-
Scrutinizing concurrent and mutually exclusive insertions in clonal cell populations of tumours or in single cells of a tumour in combination with high-quality expression profiling and proteomics analysis may teach us about underlying mechanisms of tumour heterogeneity and their role in tumour initiation and maintenance.
Abstract
Retroviral insertional mutagenesis screens have been used for many years as a tool for cancer gene discovery. In recent years, completion of the mouse genome sequence as well as improved technologies for cloning and sequencing of retroviral insertions have greatly facilitated the retrieval of more complete data sets from these screens. The concomitant increase of the size of the screens allows researchers to address new questions about the genes and signalling networks involved in tumour development. In addition, the development of new insertional mutagenesis tools such as DNA transposons enables screens for cancer genes in tissues that previously could not be analysed by retroviral insertional mutagenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Uren, A. G., Kool, J., Berns, A. & van Lohuizen, M. Retroviral insertional mutagenesis: past, present and future. Oncogene 24, 7656–7672 (2005). This review provides an historical overview of retroviral insertional mutagenesis screens and the different mechanisms in which genes can be mutated by retroviral integrations.
Jonkers, J. & Berns, A. Retroviral insertional mutagenesis as a strategy to identify cancer genes. Biochim. Biophys. Acta 1287, 29–57 (1996).
Uren, A. G. et al. Large-scale mutagenesis in p19ARF- and p53-deficient mice identifies cancer genes and their collaborative networks. Cell 133, 727–741 (2008).
Jonkers, J., Korswagen, H. C., Acton, D., Breuer, M. & Berns, A. Activation of a novel proto-oncogene, Frat1, contributes to progression of mouse T-cell lymphomas. EMBO J. 16, 441–450 (1997).
Nihrane, A., Fujita, K., Willey, R., Lyu, M. S. & Silver, J. Murine leukemia virus envelope protein in transgenic-mouse serum blocks infection in vitro. J. Virol. 70, 1882–1889 (1996).
Ikeda, H. & Sugimura, H. Fv-4 resistance gene: a truncated endogenous murine leukemia virus with ecotropic interference properties. J. Virol. 63, 5405–5412 (1989).
Ott, D. & Rein, A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J. Virol. 66, 4632–4638 (1992).
Heidmann, T., Heidmann, O. & Nicolas, J. F. An indicator gene to demonstrate intracellular transposition of defective retroviruses. Proc. Natl Acad. Sci. USA 85, 2219–2223 (1988).
Tchenio, T. & Heidmann, T. Defective retroviruses can disperse in the human genome by intracellular transposition. J. Virol. 65, 2113–2118 (1991).
Dzuris, J. L., Zhu, W., Kapkov, D., Golovkina, T. V. & Ross, S. R. Expression of mouse mammary tumour virus envelope protein does not prevent superinfection in vivo or in vitro. Virology 263, 418–426 (1999).
Suzuki, T., Minehata, K., Akagi, K., Jenkins, N. A. & Copeland, N. G. Tumour suppressor gene identification using retroviral insertional mutagenesis in Blm-deficient mice. EMBO J. 25, 3422–3431 (2006).
Suzuki, T. et al. New genes involved in cancer identified by retroviral tagging. Nature Genet. 32, 166–174 (2002). An elegant study in which a hypomorphic Blm allele is used to induce LOH of retrovirally inactivated tumour suppressor genes.
Stewart, M. et al. Insertional mutagenesis reveals progression genes and checkpoints in MYC/Runx2 lymphomas. Cancer Res. 67, 5126–5133 (2007).
Mikkers, H. et al. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nature Genet. 32, 153–159 (2002).
Erkeland, S. J. et al. Large-scale identification of disease genes involved in acute myeloid leukemia. J. Virol. 78, 1971–1980 (2004).
Lund, A. H. et al. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nature Genet. 32, 160–165 (2002).
Theodorou, V. et al. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer. Nature Genet. 39, 759–769 (2007). The first large-scale MMTV insertional mutagenesis screen to identify new cancer genes in mammary tumours.
Kim, R. et al. Genome-based identification of cancer genes by proviral tagging in mouse retrovirus-induced T-cell lymphomas. J. Virol. 77, 2056–2062 (2003).
Hasemann, M. S. et al. Mutation of C/EBPα predisposes to the development of myeloid leukemia in a retroviral insertional mutagenesis screen. Blood 111, 4309–4321 (2008).
Shendure, J. & Ji, H. Next-generation DNA sequencing. Nature Biotechnol. 26, 1135–1145 (2008). An introduction to, and overview of, next-generation massive parallel sequencing technologies.
Dupuy, A. J., Akagi, K., Largaespada, D. A., Copeland, N. G. & Jenkins, N. A. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 436, 221–226 (2005).
Collier, L. S., Carlson, C. M., Ravimohan, S., Dupuy, A. J. & Largaespada, D. A. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature 436, 272–276 (2005). The first two papers, which were published back-to-back, to report transposon mutagenesis systems that efficiently induce tumours in mice.
Carlson, C. M. & Largaespada, D. A. Insertional mutagenesis in mice: new perspectives and tools. Nature Rev. Genet. 6, 568–580 (2005).
Keng, V. W. et al. A conditional transposon-based insertional mutagenesis screen for genes associated with mouse hepatocellular carcinoma. Nature Biotechnol. 27, 264–274 (2009).
Starr, T. K. et al. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 323, 1747–1750 (2009).
Beverly, L. J., Felsher, D. W. & Capobianco, A. J. Suppression of p53 by Notch in lymphomagenesis: implications for initiation and regression. Cancer Res. 65, 7159–7168 (2005).
Beverly, L. J. & Capobianco, A. J. Perturbation of Ikaros isoform selection by MLV integration is a cooperative event in NotchIC-induced T cell leukemogenesis. Cancer Cell 3, 551–564 (2003).
Iwasaki, M. et al. Identification of cooperative genes for NUP98–HOXA9 in myeloid leukemogenesis using a mouse model. Blood 105, 784–793 (2005).
Castilla, L. H. et al. Identification of genes that synergize with Cbfb–MYH11 in the pathogenesis of acute myeloid leukemia. Proc. Natl Acad. Sci. USA 101, 4924–4929 (2004).
Feldman, B. J., Reid, T. R. & Cleary, M. L. Pim1 cooperates with E2a–Pbx1 to facilitate the progression of thymic lymphomas in transgenic mice. Oncogene 15, 2735–2742 (1997).
Slape, C. et al. Retroviral insertional mutagenesis identifies genes that collaborate with NUP98–HOXD13 during leukemic transformation. Cancer Res. 67, 5148–5155 (2007).
Li, J. et al. Leukaemia disease genes: large-scale cloning and pathway predictions. Nature Genet. 23, 348–353 (1999).
Erkeland, S. J. et al. Significance of murine retroviral mutagenesis for identification of disease genes in human acute myeloid leukemia. Cancer Res. 66, 622–626 (2006).
Adams, J. M. et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538 (1985).
Ellwood-Yen, K. et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumours. Cancer Cell 4, 223–238 (2003).
Jacobs, J. J. et al. Bmi-1 collaborates with c-Myc in tumourigenesis by inhibiting c-Myc- induced apoptosis via INK4a/ARF. Genes Dev. 13, 2678–2690 (1999).
van der Lugt, N. M. et al. Proviral tagging in E mu-myc transgenic mice lacking the Pim-1 proto-oncogene leads to compensatory activation of Pim-2. EMBO J. 14, 2536–2544 (1995).
Soucek, L. et al. Modelling Myc inhibition as a cancer therapy. Nature 455, 679–683 (2008).
Lazo, P. A., Lee, J. S. & Tsichlis, P. N. Long-distance activation of the Myc protooncogene by provirus insertion in Mlvi-1 or Mlvi-4 in rat T-cell lymphomas. Proc. Natl Acad. Sci. USA 87, 170–173 (1990).
Hickson, I. D. RecQ helicases: caretakers of the genome. Nature Rev. Cancer 3, 169–178 (2003).
Luo, G. et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nature Genet. 26, 424–429 (2000).
Garrett-Engele, C. M. et al. A mechanism misregulating p27 in tumours discovered in a functional genomic screen. PLoS Genet. 3, e219 (2007).
Tsihlias, J., Kapusta, L. & Slingerland, J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu. Rev. Med. 50, 401–423 (1999).
Park, M. S. et al. p27 and Rb are on overlapping pathways suppressing tumourigenesis in mice. Proc. Natl Acad. Sci. USA 96, 6382–6387 (1999).
Pinkel, D. & Albertson, D. G. Array comparative genomic hybridization and its applications in cancer. Nature Genet. 37, S11–S17 (2005).
Carrasco, D. R. et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell 9, 313–325 (2006).
Wood, L. D. et al. The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113 (2007). A paper describing mutations that were identified by sequencing a panel of human breast cancers. Multiple genes that are mutated in humans were also found to be CISs in MMTV-induced tumours in mice.
Sjoblom, T. et al. The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Jones, S. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 (2008).
Fujino, Y., Ohno, K. & Tsujimoto, H. Molecular pathogenesis of feline leukemia virus-induced malignancies: insertional mutagenesis. Vet. Immunol. Immunopathol. 123, 138–143 (2008).
Kanter, M. R., Smith, R. E. & Hayward, W. S. Rapid induction of B-cell lymphomas: insertional activation of c-myb by avian leukosis virus. J. Virol. 62, 1423–1432 (1988).
van Agthoven, T. et al. Functional identification of genes causing estrogen independence of human breast cancer cells. Breast Cancer Res. Treat. 114, 23–30 (2009).
Dorssers, L. C., van, A. T., Dekker, A., van Agthoven, T. L. & Kok, E. M. Induction of antiestrogen resistance in human breast cancer cells by random insertional mutagenesis using defective retroviruses: identification of bcar-1, a common integration site. Mol. Endocrinol. 7, 870–878 (1993). These authors demonstrate that insertional mutagenesis can be used to screen for genes that confer resistance to therapeutic agents in vivo.
van der, F. S. et al. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J. Natl Cancer Inst. 92, 120–127 (2000).
Miething, C. et al. Retroviral insertional mutagenesis identifies RUNX genes involved in chronic myeloid leukemia disease persistence under imatinib treatment. Proc. Natl Acad. Sci. USA 104, 4594–4599 (2007).
Johansson, F. K., Goransson, H. & Westermark, B. Expression analysis of genes involved in brain tumour progression driven by retroviral insertional mutagenesis in mice. Oncogene 24, 3896–3905 (2005).
Hanai, S. et al. Integration of human T-cell leukemia virus type 1 in genes of leukemia cells of patients with adult T-cell leukemia. Cancer Sci. 95, 306–310 (2004).
Killebrew, D. & Shiramizu, B. Pathogenesis of HIV-associated non-Hodgkin lymphoma. Curr. HIV Res. 2, 215–221 (2004).
Thrasher, A. J. et al. Gene therapy: X-SCID transgene leukaemogenicity. Nature 443, E5–E6; discussion E6–E7 (2006).
de Ridder, J., Uren, A., Kool, J., Reinders, M. & Wessels, L. Detecting statistically significant common insertion sites in retroviral insertional mutagenesis screens. PLoS Comput. Biol. 2, e166 (2006).
Wu, X., Li, Y., Crise, B. & Burgess, S. M. Transcription start regions in the human genome are favored targets for MLV integration. Science 300, 1749–1751 (2003).
Berry, C., Hannenhalli, S., Leipzig, J. & Bushman, F. D. Selection of target sites for mobile DNA integration in the human genome. PLoS Comput. Biol. 2, e157 (2006).
Lewinski, M. K. et al. Retroviral DNA integration: viral and cellular determinants of target-site selection. PLoS Pathog. 2, e60 (2006).
Mitchell, R. S. et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2, e234 (2004).
Felice, B. et al. Transcription factor binding sites are genetic determinants of retroviral integration in the human genome. PLoS ONE 4, e4571 (2009).
Sauvageau, M. et al. Quantitative expression profiling guided by common retroviral insertion sites reveals novel and cell type specific cancer genes in leukemia. Blood 111, 790–799 (2008).
Su, Q. et al. A DNA transposon-based approach to validate oncogenic mutations in the mouse. Proc. Natl Acad. Sci. USA 105, 19904–19909 (2008).
Wilson, M. H., Coates, C. J. & George, A. L. Jr. PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 15, 139–145 (2007).
Carlson, C. M. et al. Transposon mutagenesis of the mouse germline. Genetics 165, 243–256 (2003).
van Lohuizen, M., Breuer, M. & Berns, A. N-myc is frequently activated by proviral insertion in MuLV-induced T cell lymphomas. EMBO J. 8, 133–136 (1989).
Girard, L. & Jolicoeur, P. A full-length Notch1 allele is dispensable for transformation associated with a provirally activated truncated Notch1 allele in Moloney MuLV-infected MMTVD/myc transgenic mice. Oncogene 16, 517–522 (1998).
Weng, A. P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004).
Yang, L. T. et al. Fringe glycosyltransferases differentially modulate Notch1 proteolysis induced by Delta1 and Jagged1. Mol. Biol. Cell 16, 927–942 (2005).
Haines, N. & Irvine, K. D. Glycosylation regulates Notch signalling. Nature Rev. Mol. Cell Biol. 4, 786–797 (2003).
Moloney, D. J. et al. Fringe is a glycosyltransferase that modifies Notch. Nature 406, 369–375 (2000).
Largaespada, D. A., Shaughnessy, J. D. Jr, Jenkins, N. A. & Copeland, N. G. Retroviral integration at the Evi-2 locus in BXH-2 myeloid leukemia cell lines disrupts Nf1 expression without changes in steady-state Ras-GTP levels. J. Virol. 69, 5095–5102 (1995).
Cho, B. C. et al. Frequent disruption of the Nf1 gene by a novel murine AIDS virus-related provirus in BXH-2 murine myeloid lymphomas. J. Virol. 69, 7138–7146 (1995).
Acknowledgements
We thank A. Uren, A. Sparmann, J. Jonkers and M. van Lohuizen for discussions and critically reading the manuscript. J.K. was supported by the De Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) Genomics Programme and the Cancer Genomics Centre, which is supported by the Netherlands Genomics Initiative (NGI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute Drug Dictionary
FURTHER INFORMATION
Glossary
- Viraemia
-
The continuous presence of infectious viruses in an organism.
- Viraemic phase
-
The stage of viral infection of an organism in which infectious viruses are present in the body.
- Retrotranspose
-
The integration of a retrovirus or transposon in the genomic DNA of a host cell through an RNA intermediate.
- Massive parallel sequencing
-
Next-generation sequencing technologies that are able to sequence millions of DNA fragments in parallel.
- DNA transposon system
-
DNA transposons are genetic elements that can be excised from a genome or episomal element and integrate (transpose) into another DNA site. The transposase protein is required for this process.
- Gene trap
-
A vector element consisting of a splice acceptor site and polyadenylation signal that is intended to create a fusion of the cellular transcript and vector sequences, resulting in termination of transcription of the cellular gene.
Rights and permissions
About this article
Cite this article
Kool, J., Berns, A. High-throughput insertional mutagenesis screens in mice to identify oncogenic networks. Nat Rev Cancer 9, 389–399 (2009). https://doi.org/10.1038/nrc2647
Issue Date:
DOI: https://doi.org/10.1038/nrc2647
This article is cited by
-
Exploring liver cancer biology through functional genetic screens
Nature Reviews Gastroenterology & Hepatology (2021)
-
PiggyBac transposon tools for recessive screening identify B-cell lymphoma drivers in mice
Nature Communications (2019)
-
Genome-wide transposon screening and quantitative insertion site sequencing for cancer gene discovery in mice
Nature Protocols (2017)
-
Vector Integration Sites Identification for Gene-Trap Screening in Mammalian Haploid Cells
Scientific Reports (2017)
-
IRS4 induces mammary tumorigenesis and confers resistance to HER2-targeted therapy through constitutive PI3K/AKT-pathway hyperactivation
Nature Communications (2016)