Key Points
-
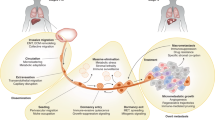
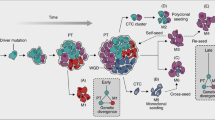
Metastasis accounts for the preponderance of morbidity and mortality in cancer, and accumulating evidence suggests that dissemination of tumour cells may occur earlier in the development of cancer than previously appreciated.
-
Metastasis is regulated either positively or negatively by proteins that promote steps in the metastatic cascade or those that suppress them.
-
Metastasis suppressor genes encode proteins that prevent or reduce the development of metastases in vivo, without simultaneously affecting primary tumour growth. This is in contrast to tumour suppressors, which affect primary tumorigenesis.
-
Metastasis suppressor proteins are lost primarily during cancer progression and not during transformation.
-
Until recently, relatively few metastasis suppressor genes had been characterized. However, expanding genomic technology has made possible in recent years the description of many metastasis suppressor genes impinging on a wide variety of cellular processes.
-
Although metastasis suppressor genes have been shown to work at multiple steps in the process of metastasis, several have been recently demonstrated to specifically suppress the colonization step of metastasis, the process by which solitary or small clusters of tumour cells living in a second organ site (micrometastases) grow to form clinically apparent and lethal macrometastases.
-
Recent work has shown techniques that restore metastasis suppressor function. These include re-expression of the gene from the endogenous locus or by exogenous gene therapy, direct administration of the protein itself and targeting important metastasis mediators downstream of the suppressor that are reciprocally induced with metastasis suppressor protein losses.
Abstract
Metastasis suppressor proteins regulate multiple steps in the metastatic cascade, including cancer cell invasion, survival in the vascular and lymphatic circulation, and colonization of distant organ sites. Understanding the biology of metastasis suppressors provides valuable mechanistic insights that may translate to therapeutic opportunities. Several reports have explored novel strategies for restoring metastasis suppressor function, including gene transfer, induction of previously suppressed gene expression and exogenous administration of gene product. Pathways activated downstream of metastasis suppressor loss can also be targeted. Although none of these strategies are yet in routine clinical use, several are being tested preclinically and in clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Fidler, I. J. & Poste, G. The “seed and soil” hypothesis revisited. Lancet Oncol. 9, 808 (2008).
Steeg, P. S. Metastasis suppressors alter the signal transduction of cancer cells. Nature Rev. Cancer 3, 55–63 (2003).
Stafford, L. J., Vaidya, K. S. & Welch, D. R. Metastasis suppressors genes in cancer. Int. J. Biochem. Cell Biol. 40, 874–891 (2008).
Tavazoie, S. F. et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 (2008).
Ma, L. & Weinberg, R. A. MicroRNAs in malignant progression. Cell Cycle 7, 570–572 (2008).
Rinker-Schaeffer, C. W., O'Keefe, J. P., Welch, D. R. & Theodorescu, D. Metastasis suppressor proteins: discovery, molecular mechanisms, and clinical application. Clin. Cancer Res. 12, 3882–3889 (2006).
Iiizumi, M., Liu, W., Pai, S. K., Furuta, E. & Watabe, K. Drug development against metastasis-related genes and their pathways: a rationale for cancer therapy. Biochim. Biophys. Acta 1786, 87–104 (2008).
Welch, D. R. Technical considerations for studying cancer metastasis in vivo. Clin. Exp. Metastasis 15, 272–306 (1997).
Eccles, S. A. & Welch, D. R. Metastasis: recent discoveries and novel treatment strategies. Lancet 369, 1742–1757 (2007).
Steeg, P. S. & Theodorescu, D. Metastasis: a therapeutic target for cancer. Nature Clin. Pract. Oncol. 5, 206–219 (2008). References 11 and 12 provide important perspectives on the rationale for targeting metastasis in human cancer, aside from the issue of suppressor genes. As developmental therapies targeting these genes must be adapted to this specialized clinical setting, such understanding is essential.
Pelkey, T. J., Frierson, H. F. Jr & Bruns, D. E. Molecular and immunological detection of circulating tumor cells and micrometastases from solid tumors. Clin. Chem. 42, 1369–1381 (1996).
Glaves, D., Huben, R. P. & Weiss, L. Haematogenous dissemination of cells from human renal adenocarcinomas. Br. J. Cancer 57, 32–35 (1988).
Fidler, I. J. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125I-5-iodo-2′-deoxyuridine. J. Natl Cancer Inst. 45, 773–782 (1970).
Alix-Panabieres, C., Riethdorf, S. & Pantel, K. Circulating tumor cells and bone marrow micrometastasis. Clin. Cancer Res. 14, 5013–5021 (2008).
Engel, J. et al. The process of metastasisation for breast cancer. Eur. J. Cancer 39, 1794–1806 (2003).
Butler, T. P. & Gullino, P. M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 35, 512–516 (1975).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008). Reference 19, coupled with the above reports on tumour cell dissemination, is a watershed in our understanding of how widely and early tumours disseminate.
Cameron, M. D. et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 60, 2541–2546 (2000).
Wong, C. W. et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 61, 333–338 (2001).
Weiss, L. Metastatic inefficiency. Adv. Cancer Res. 54, 159–211 (1990).
Steeg, P. S. Tumor metastasis: mechanistic insights and clinical challenges. Nature Med. 12, 895–904 (2006).
Weinberg, R. A. Leaving home early: reexamination of the canonical models of tumor progression. Cancer Cell 14, 283–284 (2008).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Rev. Cancer 2, 563–572 (2002).
Chambers, A. F., MacDonald, I. C., Schmidt, E. E., Morris, V. L. & Groom, A. C. Clinical targets for anti-metastasis therapy. Adv. Cancer Res. 79, 91–121 (2000).
Kauffman, E. C., Robinson, V. L., Stadler, W. M., Sokoloff, M. H. & Rinker-Schaeffer, C. W. Metastasis suppression: the evolving role of metastasis suppressor genes for regulating cancer cell growth at the secondary site. J. Urol. 169, 1122–1133 (2003).
Hedley, B. D., Allan, A. L. & Chambers, A. F. Tumor dormancy and the role of metastasis suppressor genes in regulating ectopic growth. Future Oncol. 2, 627–641 (2006).
Horak, C. E., Lee, J. H., Marshall, J. C., Shreeve, S. M. & Steeg, P. S. The role of metastasis suppressor genes in metastatic dormancy. APMIS 116, 586–601 (2008).
Steeg, P. S. et al. Evidence for a novel gene associated with low tumor metastatic potential. J. Natl Cancer Inst. 80, 200–204 (1988).
Leone, A. et al. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell 65, 25–35 (1991).
Steeg, P. S., Horak, C. E. & Miller, K. D. Clinical-translational approaches to the Nm23-H1 metastasis suppressor. Clin. Cancer Res. 14, 5006–5012 (2008).
Parhar, R. S. et al. Effects of cytokine mediated modulation of Nm23 expression on the invasion and metastatic behavior of B16F10 melanoma cells. Int. J. Cancer 60, 204–210 (1995).
Leone, A., Flatow, U., VanHoutte, K. & Steeg, P. S. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: Effects on tumor metastatic potential, colonization, and enzymatic activity. Oncogene 8, 2325–2333 (1993).
Liu, F., Zhang, Y., Zhang, X.-Y. & Chen, H.-L. Transfection of the nm23-H1 gene into human hepatocarcinoma cell line inhibits the expression of sialyl Lewis X, a 1,3 fucosyltransferase VII, and metastatic potential. J. Cancer Res. Clin. Oncol. 128, 189–196 (2002).
Hartsough, M. T. et al. Nm23-H1 metastasis suppressor phosphorylation of kinase suppressor of Ras via a histidine protein kinase pathway. J. Biol. Chem. 277, 32389–32399 (2002).
Reddy, K. B., Nabha, S. M. & Atanaskova, N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 22, 395–403 (2003).
Subramanian, C., Cotter, M. & Robertson, E. Epstein–Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm23-H1: A molecular link to cancer metastasis. Nature Med. 7, 350–355 (2001).
Kaul, R., Murakami, M., Choudhuri, T. & Robertson, E. S. Epstein–Barr virus latent nuclear antigens can induce metastasis in a nude mouse model. J. Virol. 81, 10352–10361 (2007).
Horak, C. E. et al. Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res. 67, 7238–7246 (2007).
Ouatas, T., Clare, S., Hartsough, M., DeLaRosa, A. & Steeg, P. MMTV-associated transcription factor binding sites increase nm23-H1 metastasis suppressor gene expression in human breast carcinoma cell lines. Clin. Exp. Metast. 19, 35–42 (2002).
Ouatas, T., Halverson, D. & Steeg, P. S. Dexamethasone and medroxyprogesterone acetate elevate Nm23-H1 metastasis suppressor gene expression in metastatic human breast carcinoma cells: new uses for old compounds. Clin. Cancer Res. 9, 3763–3772 (2003).
Focan, C. et al. Adjuvant high dose medroxyprogesterone acetate for early breast cancer: 13 years update in a multicentre randomized trial. Br. J. Cancer 85, 1–8 (2001).
Hupperets, P. et al. Adjuvant chemo-hormonal therapy with chclophosphamide, doxorubicin and 5-fluroruacil (CAF) with or without medroxyprogesterone acetate (MPA) for node-positive cancer patients, update at 12 years follow up. The Breast 10, 35–37 (2001).
Palmieri, D. et al. Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J. Natl Cancer Inst. 97, 632–642 (2005). Reference 45 provides an excellent example of design, execution and internal controls for preclinical studies of metastasis suppressor-inducing therapies.
Orlando, L. et al. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs 17, 961–967 (2006).
Liu, F., Qi, H.-L. & Chen, H.-L. Effects of all-trans retinoic acid and epidermal growth factor on the expression of nm23-H1 in human hepatocarcinoma cells. J. Cancer Res. Clin. Oncol. 126, 85–90 (2000).
Lin, K. H., Wang, W. J., Wu, Y. H. & Cheng, S. Y. Activation of antimetastatic Nm23-H1 gene expression by estrogen and its alpha-receptor. Endocrinology 143, 467–475 (2002).
Li, J. et al. Inhibition of ovarian cancer metastasis by adeno-associated virus-mediated gene transfer of nm23-H1 in an orthotopic transplantation model. Cancer Gene Ther. 13, 266–270 (2006).
Ichikawa, T. et al. Localization of metastasis suppressor gene(s) for prostatic cancer to the short arm of human chromosome 11. Cancer Res. 52, 3486–3490 (1992).
Ichikawa, T., Ichikawa, Y. & Isaacs, J. T. Genetic factors and suppression of metastatic ability of prostatic cancer. Cancer Res. 51, 3788–3792 (1991).
Dong, J. T. et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 268, 884–886 (1995).
Odintsova, E., Sugiura, T. & Berditchevski, F. Attenuation of EGF receptor signaling by a metastasis suppressor, the tetraspanin CD82/KAI-1. Curr. Biol. 10, 1009–1012 (2000).
Bandyopadhyay, S. et al. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nature Med. 12, 933–938 (2006).
Mashimo, T. et al. The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc. Natl Acad. Sci. USA 95, 11307–11311 (1998).
Mashimo, T. et al. Activation of the tumor metastasis suppressor gene, KAI1, by etoposide is mediated by p53 and c-Jun genes. Biochem. Biophys. Res. Commun. 274, 370–376 (2000).
Wu, Q. et al. Role of tumor metastasis suppressor gene KAI1 in digestive tract carcinomas and cancer cells. Cell Tissue Res. 314, 237–249 (2003).
Kerbel, R. S. & Kamen, B. A. The anti-angiogenic basis of metronomic chemotherapy. Nature Rev. Cancer 4, 423–436 (2004). Reference 58 reviews metronomic chemotherapy, a novel therapeutic strategy that is adaptable to simultaneous therapy with agents targeting metastasis suppressor function.
Correale, P. et al. A novel metronomic chemotherapy regimen of weekly platinum and daily oral etoposide in high-risk non-small cell lung cancer patients. Oncol. Rep. 16, 133–140 (2006).
El Touny, L. H. & Banerjee, P. P. Genistein induces the metastasis suppressor kangai-1 which mediates its anti-invasive effects in TRAMP cancer cells. Biochem. Biophys. Res. Commun. 361, 169–175 (2007).
Xu, J. H., Guo, X. Z., Ren, L. N., Shao, L. C. & Liu, M. P. KAI1 is a potential target for anti-metastasis in pancreatic cancer cells. World J. Gastroenterol. 14, 1126–1132 (2008).
Takeda, T. et al. Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res. 67, 1744–1749 (2007). References 61 and 62 are two examples of the use of viral and non-viral gene therapy strategies for reconstitution of metastasis suppressor expression.
Fu, Z. et al. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J. Natl Cancer Inst. 95, 878–889 (2003).
Yeung, K. et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401, 173–177 (1999).
Fu, Z. et al. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate 66, 248–256 (2006).
Hagan, S. et al. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin. Cancer Res. 11, 7392–7397 (2005).
Beach, S. et al. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene 27, 2243–2248 (2008).
Mai, A. The therapeutic uses of chromatin-modifying agents. Expert Opin. Ther. Targets 11, 835–851 (2007).
Kim, T. Y. et al. Transcriptional induction of DLC-1 gene through Sp1 sites by histone deacetylase inhibitors in gastric cancer cells. Exp. Mol. Med. 40, 639–646 (2008).
Bandyopadhyay, S. et al. Role of the putative tumor metastasis suppressor gene Drg-1 in breast cancer progression. Oncogene 23, 5675–5681 (2004).
Hartsough, M. et al. Elevation of breast carcinoma nm23-H1 metastasis suppressor gene expression and reduced motility by DNA methylation inhibition. Cancer Res. 61, 2320–2327 (2001).
Miyazaki, T. et al. Mutation and expression of the metastasis suppressor gene KAI1 in esophageal squamous cell carcinoma. Cancer 89, 955–962 (2000).
Kim, H. L. et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 61, 2833–2837 (2001).
Cropp, C. et al. NME1 protein expression and loss of heterozygosity mutations in primary human breast tumors. J. Natl Cancer Inst. 86, 1167–1169 (1994).
Dong, J. T. et al. Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 56, 4387–4390 (1996).
Becker, K. F. et al. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 54, 3845–3852 (1994).
Wang, L., Patel, U., Ghosh, L., Chen, H. C. & Banerjee, S. Mutation in the nm23 gene is associated with metastasis in colorectal cancer. Cancer Res. 53, 717–720 (1993).
Roth, J. A. & Cristiano, R. J. Gene therapy for cancer: what have we done and where are we going? J. Natl Cancer Inst. 89, 21–39 (1997).
Lee, J. H. et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl Cancer Inst. 88, 1731–1737 (1996).
Welch, D. R. et al. Microcell-mediated transfer of chromosome 6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene 9, 255–262 (1994).
Nash, K. T. & Welch, D. R. The KISS1 metastasis suppressor: mechanistic insights and clinical utility. Front. Biosci. 11, 647–659 (2006).
Nash, K. T. et al. Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J. Natl Cancer Inst. 99, 309–321 (2007).
Ohtaki, T. et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411, 613–617 (2001). Reference 83 is the first report of a therapeutic strategy targeting metastasis suppressor function.
Orsini, M. J. et al. Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J. Med. Chem. 50, 462–471 (2007).
Dhillo, W. S. et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 90, 6609–6615 (2005).
Thompson, E. L. et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am. J. Physiol. Endocrinol. Metab. 291, E1074–E1082 (2006).
Eckhardt, B. L. et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol. Cancer Res. 3, 1–13 (2005).
Lin, B. R. et al. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology 128, 9–23 (2005).
DerMardirossian, C. & Bokoch, G. M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 (2005).
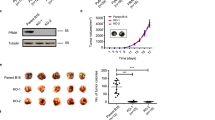
Gildea, J. J. et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 62, 6418–6423 (2002). Reference 90 provides an example of how novel high-throughput technologies have enabled identification of new metastasis suppressors.
Harding, M. A. et al. Functional genomic comparison of lineage-related human bladder cancer cell lines with differing tumorigenic and metastatic potentials by spectral karyotyping, comparative genomic hybridization, and a novel method of positional expression profiling. Cancer Res. 62, 6981–6989 (2002).
Theodorescu, D. et al. Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clin. Cancer Res. 10, 3800–3806 (2004).
Hu, L. D., Zou, H. F., Zhan, S. X. & Cao, K. M. Biphasic expression of RhoGDI2 in the progression of breast cancer and its negative relation with lymph node metastasis. Oncol. Rep. 17, 1383–1389 (2007).
Wang, Y. et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365, 671–679 (2005).
Titus, B. et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res. 65, 7320–7327 (2005).
Lalich, M., McNeel, D. G., Wilding, G. & Liu, G. Endothelin receptor antagonists in cancer therapy. Cancer Invest. 25, 785–794 (2007).
Battistini, B., Berthiaume, N., Kelland, N. F., Webb, D. J. & Kohan, D. E. Profile of past and current clinical trials involving endothelin receptor antagonists: the novel “-sentan” class of drug. Exp. Biol. Med. (Maywood) 231, 653–695 (2006).
Wu, Y. et al. Neuromedin U is regulated by the metastasis suppressor RhoGDI2 and is a novel promoter of tumor formation, lung metastasis and cancer cachexia. Oncogene 26, 765–773 (2007).
Shoemaker, R. H. The NCI60 human tumour cell line anticancer drug screen. Nature Rev. Cancer 6, 813–823 (2006).
Horak, C. E. et al. Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Res. 67, 11751–11759 (2007). Reference 100 illustrates the paradigm of using gene expression profiling of cells re-expressing metastasis suppressors to determine, and hopefully target, downstream genes that mediate the metastatic phenotype.
Ohta, H. et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 64, 994–1005 (2003).
Boucharaba, A. et al. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc. Natl Acad. Sci. USA 103, 9643–9648 (2006).
Lamb, J. et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006).
Lee, J. K. et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc. Natl Acad. Sci. USA 104, 13086–13091 (2007). References 103 and 104 provide excellent examples of the types of novel bioinformatic technologies that could enable investigators to target metastasis suppressor signatures, facilitate in silico drug discovery and ease clinical translation through patient selection and prediction of in vivo efficacy.
Potti, A. et al. Genomic signatures to guide the use of chemotherapeutics. Nature Med. 12, 1294–1300 (2006).
Kaelin, W. G. Jr. The concept of synthetic lethality in the context of anticancer therapy. Nature Rev. Cancer 5, 689–698 (2005).
Wang, H., Han, H. & Von Hoff, D. D. Identification of an agent selectively targeting DPC4 (deleted in pancreatic cancer locus 4)-deficient pancreatic cancer cells. Cancer Res. 66, 9722–9730 (2006).
Rhodes, D. R. et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6, 1–6 (2004).
Fidler, I. J. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 10, 229–243 (1991).
Ware, J. L., Paulson, D. F., Mickey, G. H. & Webb, K. S. Spontaneous metastasis of cells of the human prostate carcinoma cell line PC-3 in athymic nude mice. J. Urol. 128, 1064–1067 (1982).
Fidler, I. J. Selection of successive tumour lines for metastasis. Nat. New Biol. 242, 148–149 (1973).
Minn, A. J. et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Invest. 115, 44–55 (2005). Reference 112 is a landmark study that demonstrates empirically the exquisite tissue specificity of genes regulating metastasis.
Smith, S. C. et al. Profiling bladder cancer organ site-specific metastasis identifies LAMC2 as a novel biomarker of hematogenous dissemination. Am. J. Pathol. 174, 371–379 (2009).
Hedley, B. D. et al. BRMS1 suppresses breast cancer metastasis in multiple experimental models of metastasis by reducing solitary cell survival and inhibiting growth initiation. Clin. Exp. Metastasis 25, 727–740 (2008).
Goodison, S. et al. The RhoGAP protein DLC-1 functions as a metastasis suppressor in breast cancer cells. Cancer Res. 65, 6042–6053 (2005).
Yang, X. et al. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res. 61, 5284–5288 (2001).
Chen, S. L., Hoehne, F. M. & Giuliano, A. E. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann. Surg. Oncol. 14, 3378–3384 (2007).
Liotta, L. A. & Kohn, E. Anoikis: cancer and the homeless cell. Nature 430, 973–974 (2004).
Phadke, P. A., Vaidya, K. S., Nash, K. T., Hurst, D. R. & Welch, D. R. BRMS1 suppresses breast cancer experimental metastasis to multiple organs by inhibiting several steps of the metastatic process. Am. J. Pathol. 172, 809–817 (2008).
Lee, J. H. & Welch, D. R. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 57, 2384–2387 (1997).
Wang, J. T., Peng, D. Y., Chen, M. & Ye, J. S. Gene delivery for lung cancer using nonviral gene vectors. Pharmazie 62, 723–726 (2007).
Ohtsuka, T. et al. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene 22, 2034–2044 (2003).
Le, N. T. & Richardson, D. R. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood 104, 2967–2975 (2004).
Kurdistani, S. K. et al. Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res. 58, 4439–4444 (1998).
Bandyopadhyay, S. et al. PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in prostate and breast cancer. Cancer Res. 64, 7655–7660 (2004).
Acknowledgements
Work was supported by National Institutes of Health grant CA075115 to D.T. The authors wish to thank R. Horwitz, M. Schwartz of the University of Virginia, P. Steeg of the National Cancer Institute, and D. Welch of the University of Alabama at Birmingham for their help, suggestions and comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
ClinicalTrials.gov
National Cancer Institute Drug Dictionary
FURTHER INFORMATION
Glossary
- Metastatic colonization
-
The process by which disseminated tumour cells, present as single or small clusters of cells in a second parenchyma (micrometastasis), grow to form a clinically detectable metastatic nodule (macrometastasis).
- Epstein–Barr virus
-
(EBV). EBV is a herpes family virus that commonly causes infectious mononucleosis in humans. Infection with EBV has been implicated in Burkitt's lymphoma and nasopharyngeal carcinoma, and may also be involved in the pathogenesis of other tumours.
- Glucocorticoid response pathway
-
This nuclear hormone receptor pathway directs the regulation of genes involved in metabolism and immune function. Transcription by the glucocorticoid receptor is ligand-induced by glucocorticoid steroid hormones.
- Metronomic chemotherapy
-
Administration of chemotherapeutic drugs at comparatively low doses on a frequent or continuous schedule, with no extended interruptions, in contrast to traditional maximum tolerated dose chemotherapy.
- TRAMP
-
An autochthonous transgenic mouse model of prostate cancer. Various stages of progressive prostate disease can be observed in TRAMP mice, with focal adenocarcinomas developing between 10 and 20 weeks of age with 100% frequency.
- Mediastinal lymph node
-
Lymphatic tissue occurring in a central region of the chest between the lungs and bordered by the thoracic inlet above and the diaphragm below, where lung and other cancers frequently metastasize.
- Orthotopic xenograft
-
Establishment of a tumour by injecting human cells into the same rodent organ from which the human tumour was derived.
- Heterotopic xenograft
-
Establishment of a tumour by injecting human cells into a different rodent organ than that from which the human tumour was derived, often for technical reasons.
- Histone deacetylases
-
Enzymes that regulate chromatin structure and function through the removal of the acetyl group from the lysine residues of core nucleosomal histones, generally repressing transcription.
- DNA methyltransferases
-
Enzymes that catalyse transfer of a methyl group to cytosines in DNA, generally repressing transcription.
- Anoikis
-
Cell death in response to loss of matrix attachments.
- Osmotic pump
-
Generally small implantable pumps that use a salt concentration gradient to draw in interstitial fluid and generate pressure to expel a drug in a regulated fashion.
- Synthetic lethal
-
Describes a genetic phenomenon in which the combination of two otherwise non-lethal mutations or molecular lesions results in an unviable cell. In the case of drug discovery, the pharmaceutical agent, otherwise non-toxic, substitutes for one of these lesions and becomes lethal only in the presence of the second molecular lesion.
Rights and permissions
About this article
Cite this article
Smith, S., Theodorescu, D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer 9, 253–264 (2009). https://doi.org/10.1038/nrc2594
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2594
This article is cited by
-
An Overview on the Emerging Role of the Plasma Protease Inhibitor Protein ITIH5 as a Metastasis Suppressor
Cell Biochemistry and Biophysics (2024)
-
Mechanisms of action of NME metastasis suppressors – a family affair
Cancer and Metastasis Reviews (2023)
-
KISS1 metastasis suppressor in tumor dormancy: a potential therapeutic target for metastatic cancers?
Cancer and Metastasis Reviews (2023)
-
Metastasis suppressor genes in clinical practice: are they druggable?
Cancer and Metastasis Reviews (2023)
-
Activation of Nm23-H1 to suppress breast cancer metastasis via redox regulation
Experimental & Molecular Medicine (2021)