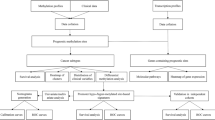
Key Points
-
The overall incidence of genitourinary cancer is rising worldwide. Even with prostate-specific antigen (PSA) testing and urine cytology, there is significant benefit to be gained from new methods for the early detection of genitourinary cancer.
-
Aberrant hypermethylation of the promoter region of genes is a frequent and early event in cancer cells. The hypermethylation is associated with loss of function of the gene.
-
Sensitive DNA-methylation-specific PCR technology permits the detection of gene methylation from rare tumour cells in tissue biopsies, urine, blood and other body fluids. Conceptually, the methylation of tumour-suppressor genes is highly specific for neoplastic cells.
-
Key studies have demonstrated sensitive and specific detection of gene methylation in urine from patients with early stage prostate, bladder or renal cancer.
-
Challenges to the clinical implementation of gene methylation-based detection include the need for validation in larger, well-defined populations with optimized and standardized methodology. Further insight into the timing of gene methylation during the earliest stages of neoplastic development will be important.
-
Future directions will probably involve screening with gene methylation for the simultaneous detection, differential diagnosis and prediction of future behaviour of several genitourinary cancer types in a single non-invasive body fluid specimen. Surveillance for the early detection of recurrence will also be a focus of study.
Abstract
DNA methylation is a common mechanism of inactivation of tumour-suppressor and other cancer genes in neoplastic cells. The advantages of gene methylation as a target for the detection and diagnosis of cancer in biopsy specimens and non-invasive body fluids such as urine or blood has led to many studies of application in genitourinary cancer. Here, we consider the background, promise and status, challenges and future directions of gene methylation and its clinical utility for the early detection of genitourinary cancer. The challenges of, and strategies for, advancing gene-methylation-based detection are relevant to all types of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal, A. et al. Cancer statistics, 2007. CA Cancer J. Clin. 57, 43–66 (2007).
Parkin, D. M. Global cancer statistics in the year 2000. Lancet Oncol. 2, 533–543 (2001).
Mackay, J. & Eriksen, M. The Tobacco Atlas. WHO [online], (2002).
Cohen, H. T. & McGovern, F. J. Renal-cell carcinoma. N. Engl. J. Med. 353, 2477–2490 (2005).
Bosl, G. J. & Motzer, R. J. Testicular germ-cell cancer. N. Engl. J. Med. 337, 242–253 (1997).
Vogelstein, B. & Kinzler, K. W. Cancer genes and the pathways they control. Nature Med. 10, 789–799 (2004).
Sidransky, D. Emerging molecular markers of cancer. Nature Rev. Cancer 2, 210–219 (2002).
Sidransky, D. Nucleic acid-based methods for the detection of cancer. Science 278, 1054–1058 (1997).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Baselga, J. et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 14, 737–744 (1996).
Cairns, P. & Sidransky, D. Molecular methods for the diagnosis of cancer. Biochim. Biophys. Acta 1423, C11–C18 (1999).
Baylin, S. B., Herman, J. G., Graff, J. R., Vertino, P. M. & Issa, J.-P. J. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv. Cancer Res. 72, 141–196 (1998).
Jones, P. A. & Laird, P. W. Cancer epigenetics comes of age. Nature Genet. 21, 163–167 (1999).
Esteller, M., Corn, P. G., Baylin, S. B. & Herman, J. G. A gene hypermethylation profile of human cancer. Cancer Res. 61, 3225–3229 (2001). The first profile of patterns of gene methylation across different cancer types.
Laird, P. W. The power and the promise of DNA methylation markers. Nature Rev. Cancer 3, 253–266 (2003).
Baylin, S. B., Belinsky, S. A. & Herman, J. G. Aberrant methylation of gene promoters in cancer- concepts, misconcepts, and promise. J. Natl Cancer Inst. 92, 1460–1461 (2000).
Maruyama, R. et al. Aberrant promoter methylation profile of bladder cancer and its relationship to clinicopathological features. Cancer Res. 61, 8659–8663 (2001).
Maruyama, R. et al. Aberrant promoter methylation profile of prostate cancers and its relationship to clinicopathological features. Clin. Cancer Res. 8, 514–519 (2002).
Jeronimo, C. et al. A quantitative promoter methylation profile of prostate cancer. Clin. Cancer Res. 10, 8472–8478 (2004).
Dulaimi, E. et al. Promoter Hypermethylation Profile of Kidney Cancer. Clin. Cancer Res. 10, 3972–3979 (2004).
Gonzalgo, M. L. et al. Molecular profiling and classification of sporadic renal cell carcinoma by quantitative methylation analysis. Clin. Cancer Res. 10, 7276–7283 (2004).
Catto, J. W. et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J. Clin. Oncol. 23, 2903–2910 (2005).
Huang, T. H., Perry, M. R. & Laux, D. E. Methylation profiling of CpG islands in human breast cancer cells. Hum. Mol. Genet. 8, 459–470 (1999).
Costello, J. F. et al. Aberrant CpG-island methylation has non-random and tumour-type–specific patterns. Nature Genet. 25, 132–138 (2000).
Yuan, E. et al. A single nucleotide polymorphism chip-based method for combined genetic and epigenetic profiling: validation in decitabine therapy and tumor/normal comparisons. Cancer Res. 66, 3443–3451 (2006). Three methodologies for global discovery of methylated genes in cancer.
Liang, G., Gonzales, F. A., Jones, P. A., Orntoft, T. F. & Thykjaer, T. Analysis of gene induction in human fibroblasts and bladder cancer cells exposed to the methylation inhibitor 5-aza-2′-deoxycytidine. Cancer Res. 62, 961–966 (2002).
Lodygin, D., Epanchintsev, A., Menssen, A., Diebold, J. & Hermeking, H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 65, 4218–4227 (2005).
Ibanez de Caceres, I. et al. Identification of novel target genes by an epigenetic reactivation screen of renal cancer. Cancer Res. 66, 5021–5028 (2006). Three global screens of novel gene methylation identified by the reactivation of gene expression through a demethylating drug in genitourinary tumour cell lines.
Herman, J. G., Graff, J. R., Myöhänen, S., Nelkin, B. D. & Baylin, S. B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl Acad. Sci. USA 93, 9821–9826 (1996). This paper introduced the now widely used MSP analysis of methylation status of a gene.
Lee, W.-H. et al. Cytidine methylation of regulatory sequences near the π-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc. Natl Acad. Sci. USA 91, 11733–11737 (1994).
Brooks, J. D. et al. CG island methylation changes near the GSTP1 gene in prostatic intraepithelial neoplasia. Cancer Epidemiol. Biomark. Prev. 7, 531–536 (1998).
Millar, D. S. et al. Detailed methylation analysis of the glutathione S-transferase pi (GSTP1) gene in prostate cancer. Oncogene 18, 1313–1324 (1999).
Cairns, P. et al. Molecular detection of early stage prostate cancer in urine. Proc. Amer. Assoc. Cancer Res. 41, 38 (2000).
Cairns, P. et al. Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin. Cancer Res. 7, 2727–2730 (2001). This exploratory study was the first demonstration of the feasibility of methylation-based detection of cancer in urine.
Goessl, C. et al. Fluorescent methylation-specific polymerase chain reaction for DNA-based detection of prostate cancer in bodily fluids. Cancer Res. 60, 5941–5945 (2000).
Goessl, C. et al. DNA-based detection of prostate cancer in urine after prostatic massage. Urology 58, 335–338 (2001).
Gonzalgo, M. L., Nakayama, M., Lee, S. M., De Marzo, A. M. & Nelson, W. G. Detection of GSTP1 methylation in prostatic secretions using combinatorial MSP analysis. Urology 63, 414–418 (2004).
Crocitto, L. E. et al. Prostate cancer molecular markers GSTP1 and hTERT in expressed prostatic secretions as predictors of biopsy results. Urology 64, 821–825 (2004).
Sathyanarayana, U. G. et al. Aberrant promoter methylation of laminin-5-encoding genes in prostate cancers and its relationship to clinicopathological features. Clin. Cancer Res. 9, 6395–6400 (2003).
Suzuki, M. et al. Methylation of apoptosis related genes in the pathogenesis and prognosis of prostate cancer. Cancer Lett. 242, 222–230 (2006).
Hoque, M. O. et al. Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects. J. Clin. Oncol. 23, 6569–6575 (2005).
Kagan, J., Srivastava, S., Barker, P. E., Belinsky, S. A. & Cairns, P. Towards clinical application of methylated DNA sequences as cancer biomarkers: a joint NCI's EDRN & NIST workshop on standards, methods, assays, reagents and tools (SMART). Cancer Res. 67, 4545–4549 (2007).
Jeronimo, C. et al. Quantitation of GSTP1 methylation in non-neoplastic prostatic tissue and organ-confined prostate adenocarcinoma. J. Natl Cancer Inst. 93, 1747–1752 (2001).
Harden, S. V., Guo, Z., Epstein, J. I. & Sidransky, D. Quantitative GSTP1 methylation clearly distinguishes benign prostatic tissue and limited prostate adenocarcinoma. J. Urol. 169, 1138–1142 (2003).
Harden, S. V. et al. Quantitative GSTP1 methylation and the detection of prostate adenocarcinoma in sextant biopsies. J. Natl Cancer Inst. 95, 1634–1637 (2003).
Tokumaru, Y. et al. Optimal use of a panel of methylation markers with GSTP1 hypermethylation in the diagnosis of prostate adenocarcinoma. Clin. Cancer Res. 10, 5518–5522 (2004).
Goessl, C. et al. Methylation-specific PCR for detection of neoplastic DNA in biopsy washings. J. Pathol. 196, 331–334 (2002).
Roehl, K. A., Antenor, J. A. & Catalona, W. J. Serial biopsy results in prostate cancer screening study. J. Urol. 167, 2435–2439 (2002).
Gonzalgo, M. L., Pavlovich, C. P., Lee, S. M. & Nelson, W. G. Prostate cancer detection by GSTP1 methylation analysis of postbiopsy urine specimens. Clin. Cancer Res. 9, 2673–2677 (2003).
Chan, M. W. et al. Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients. Clin. Cancer Res. 8, 464–470 (2002).
Dulaimi, E., Uzzo, R. G., Greenberg, R. E., Al-Saleem, T. & Cairns, P. Detection of bladder cancer in urine by a tumor suppressor gene hypermethylation panel. Clin. Cancer Res. 10, 1887–1893 (2004). References 50 and 51 were the first studies of the feasibility of methylation-based detection of bladder cancer in urine. Both compared urine cytology results and reported a higher sensitivity by gene methylation analysis.
Friedrich, M. G. et al. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clin. Cancer Res. 10, 7457–7465 (2004).
Sathyanarayana, U. G. et al. Molecular detection of noninvasive and invasive bladder tumor tissues and exfoliated cells by aberrant promoter methylation of laminin-5 encoding genes. Cancer Res. 64, 1425–1430 (2004).
Urakami, S. et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin. Cancer Res. 12, 2109–2116 (2006).
Hoque, M. O. et al. Quantitation of promoter methylation of multiple genes in urine DNA and bladder cancer detection. J. Natl Cancer Inst. 98, 996–1004 (2006).
Carmack, A. J. & Soloway, M. S. The diagnosis and staging of bladder cancer: from RBCs to TURs. Urology 67, 3–8 (2006).
Lynch, H. T. et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 104, 1535–1549 (1993).
Zambrano, N. R., Lubensky, I. A., Merino, M. J., Linehan, W. M. & Walther, M. M. Histopathology and molecular genetics of renal tumors: toward unification of a classification system. J. Urol. 162, 1246–1258 (1999).
Battagli, C. et al. Promoter hypermethylation of tumor suppressor genes in urine from kidney cancer patients. Cancer Res. 63, 8695–8699 (2003).
Hoque, M. O. et al. Quantitative detection of promoter hypermethylation of multiple genes in the tumor, urine, and serum DNA of patients with renal cancer. Cancer Res. 64, 5511–5517 (2004).
Dechet, C. B. et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J. Urol. 169, 71–74 (2003).
Honorio, S. et al. Frequent epigenetic inactivation of the RASSF1A tumour suppressor gene in testicular tumours and distinct methylation profiles of seminoma and nonseminoma testicular germ cell tumours. Oncogene 22, 461–466 (2003).
Kawakami, T., Okamoto, K., Ogawa, O. & Okada, Y. XIST unmethylated DNA fragments in male-derived plasma as a tumour marker for testicular cancer. Lancet 363, 40–42 (2004).
Ransohoff, D. F. Rules of evidence for cancer molecular-marker discovery and validation. Nature Rev. Cancer 4, 309–314 (2004).
Pepe, M. S. et al. Phases of biomarker development for early detection of cancer. J. Natl Cancer Inst. 93, 1054–1061 (2001). An important position paper because the criteria for biomarkers of early detection are less well-developed than for therapy.
Esteller, M. et al. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum. Mol. Genet. 10, 3001–3007 (2001).
Boland, C. R. et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 58, 5248–5257 (1998).
Gitan, R. S., Shi, H., Chen, C. M., Yan, P. S. & Huang, T. H. Methylation-specific oligonucleotide microarray: a new potential for high-throughput methylation analysis. Genome Res. 12, 158–164 (2002).
Fackler, M. J. et al. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 64, 4442–4452 (2004).
Eads, C. A. et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 28, E32 (2000).
Usadel, H. et al. Quantitative adenomatous polyposis coli promoter methylation analysis in tumor tissue, serum, and plasma DNA of patients with lung cancer. Cancer Res. 62, 371–375 (2002).
Diehl, F. et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl Acad. Sci. USA 102, 16368–16373 (2005).
Jahr, S. et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 61, 1659–1665 (2001).
Ferrari, M. Cancer nanotechnology: opportunities and challenges. Nature Rev. Cancer 5, 161–171 (2005).
Rideout, W. M. III et al. Progressive increases in the methylation status and heterochromatinization of the myoD CpG island during oncogenic transformation. Mol. Cell Biol. 14, 6143–6152 (1994).
Ahuja, N., Li, Q., Mohan, A. L., Baylin, S. B. & Issa, J. P. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 58, 5489–5494 (1998). A key report of age-related gene methylation in normal cells.
Brabender, J. et al. Adenomatous polyposis coli gene promoter hypermethylation in non-small cell lung cancer is associated with survival. Oncogene 20, 3528–3532 (2001). The first study to propose and demonstrate the use of empirical cut-off levels of methylation of a gene between normal, benign and cancer cells.
Hanson, J. A. et al. Gene promoter methylation in prostate tumor-associated stromal cells. J. Natl Cancer Inst. 98, 255–261 (2006).
Lehmann, U. et al. Quantitative assessment of promoter hypermethylation during breast cancer development. Am. J. Pathol. 160, 605–612 (2002).
Yan, P. S. et al. Differential distribution of DNA methylation within the RASSF1A CpG island in breast cancer. Cancer Res. 63, 6178–6186 (2003). References 78–80 are well-designed studies that considered the physical proximity of normal-appearing cells to the tumour, and used appropriate quantitative technology for the analysis of gene methylation.
Epstein, J. I. & Herawi, M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J. Urol. 175, 820–834 (2006).
Machida, E. O. et al. Hypermethylation of ASC/TMS1 is a sputum marker for late-stage lung cancer. Cancer Res. 66, 6210–6218 (2006).
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. Nature Rev. Genet. 3, 415–428 (2002).
Linehan, W. M., Walther, M. M. & Zbar, B. The genetic basis of cancer of the kidney. J. Urol. 170, 2163–2172 (2003).
Morrissey, C. et al. Epigenetic inactivation of the RASSF1A 3p21. 3 tumor suppressor gene in both clear cell and papillary renal cell carcinoma. Cancer Res. 61, 7277–7281 (2001).
Morris, M. R. et al. Tumor suppressor activity and epigenetic inactivation of hepatocyte growth factor activator inhibitor type 2/SPINT2 in papillary and clear cell renal cell carcinoma. Cancer Res. 65, 4598–4606 (2005).
Dulaimi, E. et al. Monitoring of tumor suppressor gene methylation in follow-up renal cancer patients. Proc. Amer. Assoc. Cancer Res. 46 Abst. 3152 (2005).
Bastian, P. J. et al. Preoperative serum DNA GSTP1 CpG island hypermethylation and the risk of early prostate-specific antigen recurrence following radical prostatectomy. Clin. Cancer Res. 11, 4037–4043 (2005).
Bastian, P. J. et al. Diagnostic and prognostic information in prostate cancer with the help of a small set of hypermethylated gene loci. Clin. Cancer Res. 11, 4097–4106 (2005).
Rosenbaum, E. et al. Promoter hypermethylation as an independent prognostic factor for relapse in patients with prostate cancer following radical prostatectomy. Clin. Cancer Res. 11, 8321–8325 (2005).
Fiegl, H. et al. Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 65, 1141–1145 (2005).
Gore, S. D. et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 66, 6361–6369 (2006).
Schroder, F. H. et al. Prostate cancer detection at low prostate specific antigen. J. Urol. 163, 806–812 (2000).
Catalona, W. J. et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J. Urol. 151, 1283–1290 (1994).
Sarma, A. V. & Schottenfeld, D. Prostate cancer incidence, mortality, and survival trends in the United States: 1981–2001. Semin. Urol. Oncol. 20, 3–9 (2002).
Bartsch, G. et al. Prostate cancer mortality after introduction of prostate-specific antigen mass screening in the Federal State of Tyrol, Austria. Urology 58, 417–424 (2001).
Crawford, E. D., Leewansangtong, S., Goktas, S., Holthaus, K. & Baier, M. Efficiency of prostate-specific antigen and digital rectal examination in screening, using 4. 0 ng/ml and age-specific reference range as a cutoff for abnormal values. Prostate 38, 296–302 (1999).
Herr, H., Lamm, D. L. & Denis, L. in Principles and Practice of Genitourinary Oncology ch. 26 (ed. Raghavan, D.) 273–280 (Lippincott Williams & Wilkins, 1997).
Botteman, M. F., Pashos, C. L., Redaelli, A., Laskin, B. & Hauser, R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics 21, 1315–1330 (2003).
Mao, L. et al. Molecular detection of primary bladder cancer by microsatellite analysis. Science 271, 659–662 (1996).
Gardiner, R. A. et al. Abnormal prostatic cells in ejaculates from men with prostatic cancer-a preliminary report. Br. J. Urol. 78, 414–418 (1996).
Wong, I. H. N. et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 59, 71–73 (1999).
Esteller, M. et al. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 59, 67–70 (1999). References 102 and 103 are the first two reports of the detection of gene methylation in blood from cancer patients.
Sanchez-Cespedes, M. et al. Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res. 60, 892–895 (2000).
Anker, P., Lyautey, J., Lederrey, C. & Stroun, M. Circulating nucleic acids in plasma or serum. Clin. Chim. Acta 313, 143–146 (2001).
Palmisano, W. A. et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 60, 5954–5958 (2000).
Ushijima, T. Detection and interpretation of altered methylation patterns in cancer cells. Nature Rev. Cancer 5, 223–231 (2005).
Zhou, M., Tokumaru, Y., Sidransky, D. & Epstein, J. I. Quantitative GSTP1 methylation levels correlate with Gleason grade and tumor volume in prostate needle biopsies. J. Urol. 171, 2195–2198 (2004).
Herman, J. G. et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc. Natl. Acad. Sci. USA 91, 9700–9704 (1994).
Herman, J. G. et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 55, 4525–4530 (1995).
Bachman, K. E. et al. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggests a suppressor role in kidney, brain, and other human cancers. Cancer Res. 59, 798–802 (1999).
Yoon, J. H., Dammann, R. & Pfeifer, G. P. Hypermethylation of the CpG island of the RASSF1A gene in ovarian and renal cell carcinomas. Int. J. Cancer 94, 212–217 (2001).
Dreijerink, K. et al. The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21. 3 is involved in kidney tumorigenesis. Proc. Natl Acad. Sci. USA 98, 7504–7509 (2001).
Takahashi, T. et al. Aberrant methylation of Reprimo in human malignancies. Int. J. Cancer 115, 503–510 (2005).
Jeronimo, C. et al. Quantitative GSTP1 hypermethylation in bodily fluids of patients with prostate cancer. Urology 60, 1131–1135 (2002).
Suh, C. I. et al. Comparison of telomerase activity and GSTP1 promoter methylation in ejaculate as potential screening tests for prostate cancer. Mol. Cell Probes 14, 211–217 (2000).
Yates, D. R. et al. Methylational urinalysis: a prospective study of bladder cancer patients and age stratified benign controls. Oncogene 25, 1984–1988 (2006).
Valenzuela, M. T. et al. Assessing the use of p16(INK4a) promoter gene methylation in serum for detection of bladder cancer. Eur. Urol. 42, 622–628 (2002).
Dominguez, G. et al. p14ARF promoter hypermethylation in plasma DNA as an indicator of disease recurrence in bladder cancer patients. Clin. Cancer Res. 8, 980–985 (2002).
Acknowledgements
The author wishes to thank colleagues in the US National Cancer Institute Early Detection Research Network, and Departments of Urology and Pathology at Fox Chase Cancer Center for discussion of some of the points raised in this Review. The author would like to apologize to investigators whose work could not be included in this review owing to space constraints. The author's work is supported by grants from the National Cancer Institute and the Flight Attendants Medical Research Institute.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Paul Cairns is a member of the scientific advisory board of OncoMethylome Sciences
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
NCI Early Detection Research Network
National Cancer Institute Surveillance Epidemiology and End Results
Glossary
- DNA methylation
-
Methylation occurs almost exclusively at cytosine in a CG dinucleotide pair. Methylation is an epigenetic alteration that does not involve a change in the base sequence of the DNA. Methylated DNA cannot be recognized by PCR unless bisulphite conversion is performed to produce sequence-specific changes in methylated compared with unmethylated cytosines.
- Genome copy
-
The amount of DNA in the genome of a single cell with a normal chromosome complement is equivalent to 6.6 pg. Each 3.3 pgs of human genomic DNA include 1 allele of a gene. This figure can be used to extrapolate the detection limits in a clinical specimen.
- Diagnostic coverage
-
The number of cases in which a target methylated gene is available for detection in a clinical specimen. The upper limit of diagnostic coverage represents the upper limit of sensitivity.
- Sensitivity and specificity
-
In a broad sense in the methylation-based detection studies discussed in the text, sensitivity refers to the number of samples the assay was able to identify as positive for gene methylation. Specificity refers to the proportion of non-neoplastic cases that are negative for gene methylation subject to the limits of the technology.
- Promoter CpG island
-
The CG dinucleotide is under-represented in the human genome. Clusters of CpG are found in the promoter regions of around 50% of human genes, and are termed CpG islands. With the exception of imprinted genes and X chromosome genes in the female cell, CpG islands are generally unmethylated in normal cells.
- Gleason score
-
This is a classification of the grade of prostate cancer based on how cells appear, and are arranged, under the microscope to a pathologist. A lower score of 2–4 indicates a well-differentiated tumour of slower growth. An intermediate score of 5–7 represents the most common grade of prostate cancer. High-grade, poorly differentiated tumours are scored 8–10 and show aggressive growth.
- Prostate-specific antigen
-
(PSA). A serine protease of the kallikrein gene family that is secreted into seminal fluid by prostatic epithelial cells and is found in the serum. As it is almost exclusively a product of prostate cells, measurement in blood has proved to be useful as a tumour marker for diagnosis of prostate cancer and monitoring the effectiveness of treatment.
- Haematuria
-
Blood in the urine is often the first sign of bladder cancer. Large amounts (macro) are visible but small amounts (micro) are found by light microscopy or by a clinical test. Most haematuria cases are not associated with bladder or other cancer.
- Urine cytology
-
A non-invasive procedure for the diagnosis of bladder cancer that involves the examination of cells in urine under a microscope.
- Quantitative real time MSP
-
This differs from MSP in that a third oligonucleotide with a fluorescent reporter is used. For cost and simplicity, an unmethylated housekeeping gene is used as an alternative control to the unmethylated version of the particular gene to be assayed. A quantitative value of the amount of gene methylation relative to the amount of control gene present in the input (template) DNA is generated.
- Sextant needle biopsies
-
Six biopsies, one from the top, middle and bottom of either side of the prostate are taken for review by a pathologist. The biopsy process can be painful, and patients with negative biopsy but persistently high PSA have repeat biopsies. A proportion of such patients have a subsequent positive biopsy.
- Cystoscopy
-
A cystoscope is a slender tube fitted with a lens and light that can be inserted through the urethra. Suspicious areas of the bladder can be biopsied for analysis by a pathologist. Definitive diagnosis of bladder cancer can be established by cystoscopy and biopsy.
- Candidate gene
-
Several genes that are not commonly inactivated by genetic mutation are transcriptionally inactivated by promoter hypermethylation. Such genes have been considered candidate tumour-suppressor genes.
- Bethesda criteria and MSI
-
At least five microsatellite markers are examined for repeat length — microsatellite instability (MSI) — in tumour DNA. The finding of two or more markers with MSI is considered diagnostic.
- Field effect
-
A region of tissue, often surrounding an obvious carcinoma, in which cells appear normal but have an underlying molecular alteration.
- Overdiagnosis
-
The diagnosis of a tumour that is unlikely to affect the natural lifespan of the patient and therefore leads the patient to undergo unnecessary treatment.
- Representative at presentation
-
Many exploratory studies include tumour tissue that can be accessed conveniently but might not necessarily be representative of the disease in general. Therefore, cases of advanced disease are over-represented. By definition, a more advanced tumour will have more gene alterations, so the frequency of methylation of a given gene in a type of cancer can be overestimated.
Rights and permissions
About this article
Cite this article
Cairns, P. Gene methylation and early detection of genitourinary cancer: the road ahead. Nat Rev Cancer 7, 531–543 (2007). https://doi.org/10.1038/nrc2170
Issue Date:
DOI: https://doi.org/10.1038/nrc2170
This article is cited by
-
Occurrence, analysis and removal of pesticides, hormones, pharmaceuticals, and other contaminants in soil and water streams for the past two decades: a review
Research on Chemical Intermediates (2022)
-
Promoter hypermethylation of tumor suppressor genes correlates with tumor grade and invasiveness in patients with urothelial bladder cancer
SpringerPlus (2014)
-
CpG methylation profiling in VHL related and VHL unrelated renal cell carcinoma
Molecular Cancer (2009)
-
Promoter methylation and the detection of breast cancer
Cancer Causes & Control (2009)
-
DNA methylation patterns in bladder cancer and washing cell sediments: a perspective for tumor recurrence detection
BMC Cancer (2008)