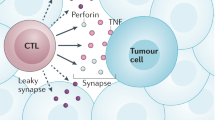
Key Points
-
A thorough review of the literature on interferon (IFN) use in cancer, using breast cancer as a case study, with discussion of the few clinical studies with sufficient numbers and sufficiently robust design to draw any conclusions. The clinical successes of IFNs (in other cancers) are often in blood-borne cancers and/or the setting of low tumour burden.
-
New understanding of the immune response to tumours and its regulation by the different types of IFN provides exciting opportunities for redesigning when and how IFNs can be used in the clinic.
-
IFNs are produced by various cell types in the tumour microenvironment, where they can have direct effects on tumour cells or indirect effects via modulation of the immune response.
-
Technology-driven improvements in measuring IFN responses via transcriptomics (and potentially proteomics) provides insights into the signal transduction pathways activated or inactivated during tumorigenesis. They also provide 'signatures' that can indicate the potential responsivity of patients to particular forms of therapy, including IFN.
-
A telling example is the discovery that tumour-cell-derived, IRF7-driven, type I IFN activates the immune system to target the process of metastasis. This paves the way for the use of IFN therapy in an adjuvant setting.
-
There are indications requiring further study that IFN may work well in combination with other immune-based therapies (for example, checkpoint inhibitors that target the programmed cell death protein PD1 or its ligand, PDL1) or hormonal therapies for which synergistic effects might be expected because components of partner pathways are themselves IFN regulated.
Abstract
The interferons (IFNs) are a family of cytokines that protect against disease by direct effects on target cells and by activating immune responses. The production and actions of IFNs are finely tuned to achieve maximal protection and avoid the potential toxicity associated with excessive responses. IFNs are back in the spotlight owing to mounting evidence that is reshaping how we can exploit this pathway therapeutically. As IFNs can be produced by, and act on, both tumour cells and immune cells, understanding this reciprocal interaction will enable the development of improved single-agent or combination therapies that exploit IFN pathways and new 'omics'-based biomarkers to indicate responsive patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hertzog, P. J. & Williams, B. R. Fine tuning type I interferon responses. Cytokine Growth Factor Rev. 24, 217–225 (2013).
Donnelly, R. P. & Kotenko, S. V. Interferon-λ: a new addition to an old family. J. Interferon Cytokine Res. 30, 555–564 (2010).
Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 (2004).
Kotenko, S. V. et al. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4, 69–77 (2003).
Fehniger, T. A. et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J. Immunol. 162, 4511–4520 (1999).
Seder, R. A., Gazzinelli, R., Sher, A. & Paul, W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl Acad. Sci. USA 90, 10188–10192 (1993).
Gough, D. J., Messina, N. L., Clarke, C. J., Johnstone, R. W. & Levy, D. E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36, 166–174 (2012).
Hwang, S. Y. et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons α and β and alters macrophage responses. Proc. Natl Acad. Sci. USA 92, 11284–11288 (1995).
LaFleur, D. W. et al. Interferon-κ, a novel type I interferon expressed in human keratinocytes. J. Biol. Chem. 276, 39765–39771 (2001).
Oritani, K. & Kanakura, Y. IFN-ζ/limitin: a member of type I IFN with mild lympho-myelosuppression. J. Cell. Mol. Med. 9, 244–254 (2005).
Fung, K. Y. et al. Interferon-ɛ protects the female reproductive tract from viral and bacterial infection. Science 339, 1088–1092 (2013).
Lutfalla, G. et al. Mutant U5A cells are complemented by an interferon-αβ receptor subunit generated by alternative processing of a new member of a cytokine receptor gene cluster. EMBO J. 14, 5100–5108 (1995).
Owczarek, C. M. et al. Cloning and characterization of soluble and transmembrane isoforms of a novel component of the murine type I interferon receptor, IFNAR 2. J. Biol. Chem. 272, 23865–23870 (1997).
Gazziola, C. et al. The relative endogenous expression levels of the IFNAR2 isoforms influence the cytostatic and pro-apoptotic effect of IFNα on pleomorphic sarcoma cells. Int. J. Oncol. 26, 129–140 (2005).
Novick, D., Cohen, B. & Rubinstein, M. Soluble interferon-α receptor molecules are present in body fluids. FEBS Lett. 314, 445–448 (1992).
Ambrus, J. L. Sr et al. Free interferon-α/β receptors in the circulation of patients with adenocarcinoma. Cancer 98, 2730–2733 (2003).
Iseda, T., Yokoyama, M., Kanayana, H., Oomoto, Y. & Kagawa, S. [An investigation of serum soluble interferon receptor levels in patients with renal cell carcinoma]. Nihon Hinyokika Gakkai Zasshi 91, 514–519 (2000).
Samarajiwa, S. A. et al. Soluble IFN receptor potentiates in vivo type I IFN signaling and exacerbates TLR4-mediated septic shock. J. Immunol. 192, 4425–4435 (2014).
Hardy, M. P. et al. The soluble murine type I interferon receptor Ifnar-2 is present in serum, is independently regulated, and has both agonistic and antagonistic properties. Blood 97, 473–482 (2001).
de Weerd, N. A. et al. Structural basis of a unique interferon-βsignaling axis mediated via the receptor IFNAR1. Nat. Immunol. 14, 901–907 (2013). This paper is the first to comprehensively describe how IFNβ can signal via a mechanism different from that of IFNα, findings backed by fine structural data, and sets the precedent for selective action of a type I IFN via differential activation of the two receptor chains.
Silvennoinen, O., Ihle, J. N., Schlessinger, J. & Levy, D. E. Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature 366, 583–585 (1993).
Platanias, L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005). This article reviews the multiple signalling pathways activated by type I and type II IFNs including classical JAK–STAT pathways and the so-called 'alternative' signalling pathways.
Cheon, H., Yang, J. & Stark, G. R. The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins. J. Interferon Cytokine Res. 31, 33–40 (2011).
Luszczek, W., Cheriyath, V., Mekhail, T. M. & Borden, E. C. Combinations of DNA methyltransferase and histone deacetylase inhibitors induce DNA damage in small cell lung cancer cells: correlation of resistance with IFN-stimulated gene expression. Mol. Cancer Ther. 9, 2309–2321 (2010).
Samarajiwa, S. A., Forster, S., Auchettl, K. & Hertzog, P. J. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res. 37, D852–D857 (2009).
Khoo, J. J., Forster, S. & Mansell, A. Toll-like receptors as interferon-regulated genes and their role in disease. J. Interferon Cytokine Res. 31, 13–25 (2011).
Porritt, R. A. & Hertzog, P. J. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 36, 150–160 (2015).
Balkwill, F., Watling, D. & Taylor-Papadimitriou, J. Inhibition by lymphoblastoid interferon of growth of cells derived from the human breast. Int. J. Cancer 22, 258–265 (1978).
Hobeika, A. C., Subramaniam, P. S. & Johnson, H. M. IFNα induces the expression of the cyclin-dependent kinase inhibitor p21 in human prostate cancer cells. Oncogene 14, 1165–1170 (1997).
Lu, M. et al. Interferon-α targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp. Hematol. 38, 472–480 (2010).
Platanias, L. C. et al. CrkL and CrkII participate in the generation of the growth inhibitory effects of interferons on primary hematopoietic progenitors. Exp. Hematol. 27, 1315–1321 (1999).
Cook, S. J., Rubinfeld, B., Albert, I. & McCormick, F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 12, 3475–3485 (1993).
Kumar, S., Fajardo, J. E., Birge, R. B. & Sriram, G. Crk at the quarter century mark: perspectives in signaling and cancer. J. Cell. Biochem. 115, 819–825 (2013).
Fathers, K. E. et al. Crk adaptor proteins act as key signaling integrators for breast tumorigenesis. Breast Cancer Res. 14, R74 (2012).
Fulda, S. & Debatin, K. M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25, 4798–4811 (2006).
Thyrell, L. et al. Mechanisms of Interferon-alpha induced apoptosis in malignant cells. Oncogene 21, 1251–1262 (2002).
Choi, E. A. et al. Stat1-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand and the cell-surface death signaling pathway by interferon beta in human cancer cells. Cancer Res. 63, 5299–5307 (2003).
Bernardo, A. R., Cosgaya, J. M., Aranda, A. & Jimenez-Lara, A. M. Synergy between RA and TLR3 promotes type I IFN-dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells. Cell Death Dis. 4, e479 (2013).
Apelbaum, A., Yarden, G., Warszawski, S., Harari, D. & Schreiber, G. Type I interferons induce apoptosis by balancing cFLIP and caspase-8 independent of death ligands. Mol. Cell. Biol. 33, 800–814 (2013).
Chawla-Sarkar, M. et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8, 237–249 (2003).
Weber, F., Kochs, G. & Haller, O. Inverse interference: how viruses fight the interferon system. Viral Immunol. 17, 498–515 (2004).
Hasthorpe, S., Holland, K., Nink, V., Lawler, C. & Hertzog, P. Mechanisms of resistance off NSCLC to interferons. Int. J. Oncol. 10, 933–938 (1997).
Wagner, T. C. et al. Interferon receptor expression regulates the antiproliferative effects of interferons on cancer cells and solid tumors. Int. J. Cancer 111, 32–42 (2004).
Levin, D., Harari, D. & Schreiber, G. Stochastic receptor expression determines cell fate upon interferon treatment. Mol. Cell. Biol. 31, 3252–3266 (2011).
Bhattacharya, S. et al. Anti-tumorigenic effects of Type 1 interferon are subdued by integrated stress responses. Oncogene 32, 4214–4221 (2013).
Bromberg, J. F., Horvath, C. M., Wen, Z., Schreiber, R. D. & Darnell, J. E. Jr. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon α and interferon γ. Proc. Natl Acad. Sci. USA 93, 7673–7678 (1996).
Levy, D. E. & Gilliland, D. G. Divergent roles of STAT1 and STAT5 in malignancy as revealed by gene disruptions in mice. Oncogene 19, 2505–2510 (2000).
Balkwill, F., Taylor-Papadimitriou, J., Fantes, K. H. & Sebesteny, A. Human lymphoblastoid interferon can inhibit the growth of human breast cancer xenografts in athymic (nude) mice. Eur. J. Cancer 16, 569–573 (1980).
Borden, E. C., Hogan, T. F. & Voelkel, J. G. Comparative antiproliferative activity in vitro of natural interferons α and β for diploid and transformed human cells. Cancer Res. 42, 4948–4953 (1982).
Fuchs, S. Y. Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J. Interferon Cytokine Res. 33, 211–225 (2013).
Cheon, H., Borden, E. C. & Stark, G. R. Interferons and their stimulated genes in the tumor microenvironment. Semin. Oncol. 41, 156–173 (2014).
Burnet, M. Cancer: a biological approach. I. The processes of control. Br. Med. J. 1, 779–786 (1957).
Burnet, F. M. Immunological surveillance in neoplasia. Transplant. Rev. 7, 3–25 (1971).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Gresser, I., Maury, C. & Brouty-Boye, D. Mechanism of the antitumour effect of interferon in mice. Nature 239, 167–168 (1972).
Dunn, G. P. et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 6, 722–729 (2005). This paper is from the Schreiber laboratory; it finds a role for type I and type II IFNs in regulating the 'immunoediting' of tumours by modulating their immune environment.
Liu, Y. J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23, 275–306 (2005).
Liu, C. et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J. Clin. Invest. 118, 1165–1175 (2008).
Treilleux, I. et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 10, 7466–7474 (2004).
Sisirak, V. et al. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 72, 5188–5197 (2012).
Bidwell, B. N. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012). This paper describes the IRF7-driven type I IFN response produced by mammary tumours that drives a systemic immune response that targets metastatic cells, the IRF7 gene signature that is indicative of patient responses, and then points to a potential new situation for using IFN as adjuvant therapy to prevent metastasis in prescreened patients.
Greiner, J. W. et al. Enhanced expression of surface tumor-associated antigens on human breast and colon tumor cells after recombinant human leukocyte α-interferon treatment. Cancer Res. 44, 3208–3214 (1984).
Boyer, C. M. et al. Differential induction by interferons of major histocompatibility complex-encoded and non-major histocompatibility complex-encoded antigens in human breast and ovarian carcinoma cell lines. Cancer Res. 49, 2928–2934 (1989).
Schiavoni, G., Mattei, F. & Gabriele, L. Type I interferons as stimulators of DC-mediated cross-priming: impact on anti-tumor response. Front. Immunol. 4, 483 (2013).
Joffre, O. P., Segura, E., Savina, A. & Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 12, 557–569 (2012).
Curtsinger, J. M. & Mescher, M. F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 22, 333–340 (2010).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Pace, L. et al. APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. J. Immunol. 184, 5969–5979 (2010).
Srivastava, S., Koch, M. A., Pepper, M. & Campbell, D. J. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J. Exp. Med. 211, 961–974 (2014).
Zoglmeier, C. et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin. Cancer Res. 17, 1765–1775 (2011).
Wang, Z. K. et al. Regulatory T cells increase in breast cancer and in stage IV breast cancer. Cancer Immunol. Immunother. 61, 911–916 (2012).
Watanabe, M. A., Oda, J. M., Amarante, M. K. & Cesar Voltarelli, J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 29, 569–579 (2010).
Duluc, D. et al. Interferon-γ reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int. J. Cancer 125, 367–373 (2009).
Propper, D. J. et al. Low-dose IFN-γ induces tumor MHC expression in metastatic malignant melanoma. Clin. Cancer Res. 9, 84–92 (2003).
Boehm, U., Klamp, T., Groot, M. & Howard, J. C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15, 749–795 (1997).
Schreiner, B. et al. Interferon-β enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J. Neuroimmunol. 155, 172–182 (2004).
Eppihimer, M. J. et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9, 133–145 (2002).
Mattei, F., Schiavoni, G., Belardelli, F. & Tough, D. F. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 167, 1179–1187 (2001).
Anthony, S. M., Howard, M. E., Hailemichael, Y., Overwijk, W. W. & Schluns, K. S. Soluble interleukin-15 complexes are generated in vivo by type I interferon dependent and independent pathways. PLoS ONE 10, e0120274 (2015).
Burkett, P. R. et al. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 200, 825–834 (2004).
Sathe, P. et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat. Commun. 5, 4539 (2014).
Waldmann, T. A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol. Res. 3, 219–227 (2015).
Nguyen, K. B. et al. Coordinated and distinct roles for IFN-αβ, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169, 4279–4287 (2002).
Herberman, R. B., Ortaldo, J. R., Rubinstein, M. & Pestka, S. Augmentation of natural and antibody-dependent cell-mediated cytotoxicity by pure human leukocyte interferon. J. Clin. Immunol. 1, 149–153 (1981).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Lanier, L. L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Fregni, G., Perier, A., Avril, M. F. & Caignard, A. NK cells sense tumors, course of disease and treatments: consequences for NK-based therapies. Oncoimmunology 1, 38–47 (2012).
Swann, J. B. et al. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 178, 7540–7549 (2007).
Gresser, I., Belardelli, F., Maury, C., Maunoury, M. T. & Tovey, M. G. Injection of mice with antibody to interferon enhances the growth of transplantable murine tumors. J. Exp. Med. 158, 2095–2107 (1983).
Deonarain, R. et al. Critical roles for IFN-β in lymphoid development, myelopoiesis, and tumor development: links to tumor necrosis factor alpha. Proc. Natl Acad. Sci. USA 100, 13453–13458 (2003).
Koromilas, A. E. & Sexl, V. The tumor suppressor function of STAT1 in breast cancer. JAKSTAT 2, e23353 (2013).
Kaplan, D. H. et al. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA 95, 7556–7561 (1998).
Bi, X. et al. Loss of interferon regulatory factor 5 (IRF5) expression in human ductal carcinoma correlates with disease stage and contributes to metastasis. Breast Cancer Res. 13, R111 (2011).
Wong, L. H. et al. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3γ. J. Biol. Chem. 272, 28779–28785 (1997).
Landolfo, S. et al. Chronic myeloid leukemia cells resistant to interferon-α lack STAT1 expression. Hematol. J. 1, 7–14 (2000).
Slattery, M. L., Lundgreen, A., Bondurant, K. L. & Wolff, R. K. Interferon-signaling pathway: associations with colon and rectal cancer risk and subsequent survival. Carcinogenesis 32, 1660–1667 (2011).
Stiff, A. & Carson, W. III. Investigations of interferon-λ for the treatment of cancer. J. Innate Immun. 7, 243–250 (2015).
De Lustig, E. S., Cortade de De La Pena, N. & Canonico, A. Interferon in breast cancer. Tumori 63, 155–162 (1977).
Widschwendter, A. et al. Prognostic significance of signal transducer and activator of transcription 1 activation in breast cancer. Clin. Cancer Res. 8, 3065–3074 (2002).
Sun, Y., Yang, S., Sun, N. & Chen, J. Differential expression of STAT1 and p21 proteins predicts pancreatic cancer progression and prognosis. Pancreas 43, 619–623 (2014).
Ahtiainen, L. et al. Defects in innate immunity render breast cancer initiating cells permissive to oncolytic adenovirus. PLoS ONE 5, e13859 (2010).
Platanias, L. C. Interferons and their antitumor properties. J. Interferon Cytokine Res. 33, 143–144 (2013).
Stein, B. L. & Tiu, R. V. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J. Interferon Cytokine Res. 33, 145–153 (2013).
Hervas-Stubbs, S. et al. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 17, 2619–2627 (2011).
Talpaz, M., Hehlmann, R., Quintas-Cardama, A., Mercer, J. & Cortes, J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia 27, 803–812 (2013).
Ascierto, P. A. et al. Interferon α for the adjuvant treatment of melanoma: review of international literature and practical recommendations from an expert panel on the use of interferon. J. Chemother. 26, 193–201 (2014). Reference 106 is a review of the international literature and practical recommendations on the use of IFN from an expert panel.
Kirkwood, J. M. et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB–III melanoma: results of intergroup trial E1694/S9512/C509801. J. Clin. Oncol. 19, 2370–2380 (2001).
Mocellin, S., Pasquali, S., Rossi, C. R. & Nitti, D. Interferon α adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J. Natl Cancer Inst. 102, 493–501 (2010). References 107 and 108 are a primary paper, review and meta-studies that confirm the efficacy of adjuvant high-dose IFNα therapy in patients with high-risk melanoma.
Pasquali, S. & Mocellin, S. The anticancer face of interferon α (IFN-α): from biology to clinical results, with a focus on melanoma. Curr. Med. Chem. 17, 3327–3336 (2010).
Akman, T. et al. Long-term outcomes and prognostic factors of high-risk malignant melanoma patients after surgery and adjuvant high-dose interferon treatment: a single-center experience. Chemotherapy 60, 228–238 (2014).
Kilbridge, K. L. et al. Patient preferences for adjuvant interferon α-2b treatment. J. Clin. Oncol. 19, 812–823 (2001).
de La Salmoniere, P., Grob, J. J., Dreno, B., Delaunay, M. & Chastang, C. White blood cell count: a prognostic factor and possible subset indicator of optimal treatment with low-dose adjuvant interferon in primary melanoma. Clin. Cancer Res. 6, 4713–4718 (2000).
Colombo, N. et al. Anti-tumor and immunomodulatory activity of intraperitoneal IFN-γ in ovarian carcinoma patients with minimal residual tumor after chemotherapy. Int. J. Cancer 51, 42–46 (1992).
Veronese, F. M. & Mero, A. The impact of PEGylation on biological therapies. BioDrugs 22, 315–329 (2008).
Tseng, T. C., Kao, J. H. & Chen, D. S. Peginterferon α in the treatment of chronic hepatitis B. Expert Opin. Biol. Ther. 14, 995–1006 (2014).
Druyts, E. et al. Efficacy and safety of pegylated interferon alfa-2a or alfa-2b plus ribavirin for the treatment of chronic hepatitis C in children and adolescents: a systematic review and meta-analysis. Clin. Infect. Dis. 56, 961–967 (2013).
Eggermont, A. M. et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 372, 117–126 (2008).
Eggermont, A. M. et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J. Clin. Oncol. 30, 3810–3818 (2012).
Daud, A. et al. Management of pegylated interferon α toxicity in adjuvant therapy of melanoma. Expert Opin. Biol. Ther. 12, 1087–1099 (2012).
Rozati, S., Naef, L., Levesque, M. P., French, L. E. & Dummer, R. Real-life experience with pegylated interferon and conventional interferon in adjuvant melanoma therapy. J. Immunother. 36, 52–56 (2013).
Tarhini, A. A. & Kirkwood, J. M. How much of a good thing? What duration for interferon alfa-2b adjuvant therapy? J. Clin. Oncol. 30, 3773–3776 (2012).
Aranda, F. et al. Trial Watch: Toll-like receptor agonists in oncological indications. Oncoimmunology 3, e29179 (2014).
Qin, M., Li, Y., Yang, X. & Wu, H. Safety of Toll-like receptor 9 agonists: a systematic review and meta-analysis. Immunopharmacol. Immunotoxicol. 36, 251–260 (2014).
Hartman, L. L. et al. Pediatric phase II trials of poly-ICLC in the management of newly diagnosed and recurrent brain tumors. J. Pediatr. Hematol. Oncol. 36, 451–457 (2014).
Jeung, H. C. et al. Phase III trial of adjuvant 5-fluorouracil and adriamycin versus 5-fluorouracil, adriamycin, and polyadenylic-polyuridylic acid (polyA:U) for locally advanced gastric cancer after curative surgery: final results of 15-year follow-up. Ann. Oncol. 19, 520–526 (2008).
Kemeny, N. et al. Randomized trial of standard therapy with or without poly I:C in patients with superficial bladder cancer. Cancer 48, 2154–2157 (1981).
Gutterman, J. U. et al. Leukocyte interferon-induced tumor regression in human metastatic breast cancer, multiple myeloma, and malignant lymphoma. Ann. Intern. Med. 93, 399–406 (1980).
Borden, E. C. et al. Leukocyte-derived interferon (alpha) in human breast carcinoma. The American Cancer Society phase II trial. Ann. Intern. Med. 97, 1–6 (1982).
Sherwin, S. A. et al. A multiple-dose phase I trial of recombinant leukocyte A interferon in cancer patients. JAMA 248, 2461–2466 (1982).
Sherwin, S. A. et al. Recombinant leukocyte A interferon in advanced breast cancer. Results of a phase II efficacy trial. Ann. Intern. Med. 98, 598–602 (1983).
Muss, H. B. et al. A phase II study of recombinant alpha interferon in patients with recurrent or metastatic breast cancer. J. Clin. Oncol. 2, 1012–1016 (1984).
Nethersell, A., Smedley, H., Katrak, M., Wheeler, T. & Sikora, K. Recombinant interferon in advanced breast cancer. Br. J. Cancer 49, 615–620 (1984).
Padmanabhan, N., Balkwill, F. R., Bodmer, J. G. & Rubens, R. D. Recombinant DNA human interferon alpha 2 in advanced breast cancer: a phase 2 trial. Br. J. Cancer 51, 55–60 (1985).
Quesada, J. R. et al. Collaborative phase I–II study of recombinant DNA-produced leukocyte interferon (clone A) in metastatic breast cancer, malignant lymphoma, and multiple myeloma. Am. J. Med. 77, 427–432 (1984).
Pouillart, P. et al. Administration of fibroblast interferon to patients with advanced breast cancer: possible effects on skin metastasis and on hormone receptors. Eur. J. Cancer Clin. Oncol. 18, 929–935 (1982).
Quesada, J. R., Gutterman, J. U. & Hersh, E. M. Clinical and immunological study of beta interferon by intramuscular route in patients with metastatic breast cancer. J. Interferon Res. 2, 593–599 (1982).
Bruntsch, U., Groos, G., Tigges, F. J., Hofschneider, P. H. & Gallmeier, W. M. Lack of response in nine patients with breast cancer treated with fibroblast interferon. Cancer Chemother. Pharmacol. 13, 39–42 (1984).
Laszlo, J., Hood, L., Cox, E. & Goodwin, B. A randomized trial of low doses of alpha interferon in patients with breast cancer. J. Biol. Response Mod. 5, 206–210 (1986).
Naso, C. et al. Lymphoblastoid interferon in advanced breast cancer: a phase II study. J. Chemother. 5, 258–261 (1993).
Sarna, G. P. & Figlin, R. A. Phase II trial of alpha-lymphoblastoid interferon given weekly as treatment of advanced breast cancer. Cancer Treat. Rep. 69, 547–549 (1985).
van den Berg, H. W., Leahey, W. J., Lynch, M., Clarke, R. & Nelson, J. Recombinant human interferon alpha increases oestrogen receptor expression in human breast cancer cells (ZR-75-1) and sensitizes them to the anti-proliferative effects of tamoxifen. Br. J. Cancer 55, 255–257 (1987).
Sica, G. et al. Effect of natural beta-interferon on estrogen receptor mRNA of breast cancer cells. Anticancer Res. 12, 2061–2064 (1992).
Sica, G., Natoli, V., Stella, C. & Del Bianco, S. Effect of natural beta-interferon on cell proliferation and steroid receptor level in human breast cancer cells. Cancer 60, 2419–2423 (1987).
Seymour, L. & Bezwoda, W. R. Interferon plus tamoxifen treatment for advanced breast cancer: in vivo biologic effects of two growth modulators. Br. J. Cancer 68, 352–356 (1993).
Sica, G. et al. Steroid receptor enhancement by natural interferon-beta in advanced breast cancer. Eur. J. Cancer 29A, 329–333 (1993).
Di Martino, L., Demontis, B., Saccani Iotti, G. & Murenu, G. In vivo effect induced by interferon beta on steroid receptor status, cell kinetics and DNA ploidy in operable breast cancer patients. Anticancer Res. 15, 537–541 (1995).
Kornek, G. et al. Effect of interferon alpha-2a on hormone receptor status in patients with advanced breast cancer. Cancer Invest. 17, 189–194 (1999).
Buzzi, E. et al. Natural interferon-beta and tamoxifen in hormone-resistant patients with advanced breast cancer. Anticancer Res. 15, 2187–2190 (1995).
Buzzi, F. et al. Combination of beta-interferon and tamoxifen as a new way to overcome clinical resistance to tamoxifen in advanced breast cancer. Anticancer Res. 12, 869–871 (1992).
Miglietta, L. et al. Tamoxifen and alpha interferon in advanced breast cancer. J. Chemother. 3, 383–386 (1991).
Macheledt, J. E. et al. Phase II evaluation of interferon added to tamoxifen in the treatment of metastatic breast cancer. Breast Cancer Res. Treat. 18, 165–170 (1991).
Repetto, L. et al. Tamoxifen and interferon-beta for the treatment of metastatic breast cancer. Breast Cancer Res. Treat. 39, 235–238 (1996).
Barak, V. et al. Changes in cytokine production of breast cancer patients treated with interferons. Cytokine 10, 977–983 (1998).
Lama, G., Angelucci, C., Recchia, F. & Sica, G. Combined effects of 13-cis-retinoic acid, tamoxifen and interferon on the growth of human breast cancer cells. Cancer Lett. 100, 181–189 (1996).
Recchia, F. et al. Beta-interferon, retinoids and tamoxifen combination in advanced breast cancer. Clin. Ter. 149, 203–208 (1998).
Recchia, F. et al. Beta-interferon, retinoids and tamoxifen in metastatic breast cancer: long-term follow-up of a phase II study. Oncol. Rep. 21, 1011–1016 (2009).
Recchia, F. et al. Interferon-beta, retinoids, and tamoxifen in the treatment of metastatic breast cancer: a phase II study. J. Interferon Cytokine Res. 15, 605–610 (1995).
Chiesa, M. D. et al. Tamoxifen versus tamoxifen plus 13-cis-retinoic acid versus tamoxifen plus interferon alpha-2a as first-line endocrine treatments in advanced breast cancer: updated results of a phase II, prospective, randomised multicentre trial. Acta Biomed. 78, 204–209 (2007).
Schreiber, G. & Piehler, J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 36, 139–149 (2015).
Welander, C. E. Overview of preclinical and clinical studies of interferon alfa-2b in combination with cytotoxic drugs. Invest. New Drugs 5 (Suppl.), S47–S59 (1987).
Pronzato, P. et al. Cisplatin and recombinant alpha interferon in advanced breast cancer. Ann. Oncol. 1, 150–151 (1990).
Walters, R. S., Theriault, R. L., Booser, D. J., Esparza, L. & Hortobagyi, G. N. Phase II study of recombinant alpha-interferon (rIFN alpha) and continuous-infusion 5-fluorouracil in metastatic breast cancer. J. Immunother. Emphasis Tumor Immunol. 18, 185–187 (1995).
Iaffaioli, R. V. et al. Phase II study of high-dose epirubicin, lonidamine, alpha 2b interferon in advanced breast cancer. Breast Cancer Res. Treat. 35, 243–248 (1995).
Oldham, R. K., Blumenschein, G., Schwartzberg, L., Birch, R. & Arnold, J. Combination biotherapy utilizing interleukin-2 and alpha interferon in patients with advanced cancer: a National Biotherapy Study Group Trial. Mol. Biother. 4, 4–9 (1992).
Walters, R. S., Theriault, R. L., Holmes, F. A., Esparza, L. & Hortobagyi, G. N. Phase II study of recombinant alpha-interferon and recombinant interleukin-2 metastatic breast cancer. J. Immunother. Emphasis Tumor Immunol. 16, 303–305 (1994).
Kimmick, G. et al. Subcutaneously administered recombinant human interleukin-2 and interferon alfa-2a for advanced breast cancer: a phase II study of the Cancer and Leukemia Group B (CALGB 9041). Invest. New Drugs 22, 83–89 (2004).
Orita, K., Fuchimoto, S., Kurimoto, M., Ando, S. & Minowada, J. Early phase II study of interferon-alpha and tumor necrosis factor-alpha combination in patients with advanced cancer. Acta Med. Okayama 46, 103–112 (1992).
Nicolini, A. & Carpi, A. Beta-interferon and interleukin-2 prolong more than three times the survival of 26 consecutive endocrine dependent breast cancer patients with distant metastases: an exploratory trial. Biomed. Pharmacother. 59, 253–263 (2005).
Nicolini, A., Carpi, A. & Rossi, G. An immunotherapy schedule in endocrine-dependent metastatic breast cancer: correlation between clinical course and immunologic parameters. J. Immunother. 28, 276–279 (2005).
Nicolini, A., Carpi, A. & Rossi, G. Relationship of cellular immunity, cytokines and CRP with clinical course in breast cancer patients with endocrine-dependent distant metastases treated with immunotherapy. Cancer Lett. 251, 330–338 (2007).
Ducret, J. P. et al. A phase I clinical tolerance study of polyadenylic-polyuridylic acid in cancer patients. J. Biol. Response Mod. 4, 129–133 (1985).
Lacour, J. et al. Adjuvant treatment with polyadenylic-polyuridylic acid (Polya. Polyu) in operable breast cancer. Lancet 2, 161–164 (1980).
Lacour, J. Clinical trials using polyadenylic-polyuridylic acid as an adjuvant to surgery in treating different human tumors. J. Biol. Response Mod. 4, 538–543 (1985). References 172 and 173 describe the use of TLR agonists (double-stranded RNAs) to induce endogenous IFNs to activate antitumour immunity.
Lacour, J. et al. Adjuvant treatment with polyadenylic-polyuridylic acid in operable breast cancer: updated results of a randomised trial. Br. Med. J. (Clin. Res. Ed.) 288, 589–592 (1984).
Laplanche, A. et al. Polyadenylic-polyuridylic acid plus locoregional radiotherapy versus chemotherapy with CMF in operable breast cancer: a 14 year follow-up analysis of a randomized trial of the Federation Nationale des Centres de Lutte contre le Cancer (FNCLCC). Breast Cancer Res. Treat. 64, 189–191 (2000).
Salaun, B. et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res. 71, 1607–1614 (2011).
Rack, B. et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl Cancer Inst. 106, dju066 (2014).
Giuliano, M. et al. Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res. 16, 440 (2014).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Sun, W. H. et al. Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 91, 570–576 (1998).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Chiche, L. et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 66, 1583–1595 (2014).
Kirkwood, J. Cancer immunotherapy: the interferon-alpha experience. Semin. Oncol. 29, 18–26 (2002).
Mattarollo, S. R. et al. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 71, 4809–4820 (2011).
Zitvogel, L., Galluzzi, L., Smyth, M. J. & Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39, 74–88 (2013).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108, 7142–7147 (2011).
Hannesdottir, L. et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur. J. Immunol. 43, 2718–2729 (2013).
Slaney, C. Y., Rautela, J. & Parker, B. S. The emerging role of immunosurveillance in dictating metastatic spread in breast cancer. Cancer Res. 73, 5852–5857 (2013).
Formenti, S. C. & Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 10, 718–726 (2009).
Demaria, S., Pilones, K. A., Vanpouille-Box, C., Golden, E. B. & Formenti, S. C. The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Radiat. Res. 182, 170–181 (2014).
Naidoo, J., Page, D. B. & Wolchok, J. D. Immune modulation for cancer therapy. Br. J. Cancer 111, 2214–2219 (2014).
Hertzog, P., Forster, S. & Samarajiwa, S. Systems biology of interferon responses. J. Interferon Cytokine Res. 31, 5–11 (2011).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Schiavoni, G. et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 71, 768–778 (2011).
Yamaguchi, R. et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum. Pathol. 43, 1688–1694 (2012).
Litterman, A. J., Dudek, A. Z. & Largaespada, D. A. Alkylating chemotherapy may exert a uniquely deleterious effect upon neo-antigen-targeting anticancer vaccination. Oncoimmunology 2, e26294 (2013).
Nielsen, J. S. et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 18, 3281–3292 (2012).
de Visser, K. E., Korets, L. V. & Coussens, L. M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7, 411–423 (2005).
Olkhanud, P. B. et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 71, 3505–3515 (2011).
Watanabe, N. et al. Type I IFN-mediated enhancement of anti-leukemic cytotoxicity of γδ T cells expanded from peripheral blood cells by stimulation with zoledronate. Cytotherapy 8, 118–129 (2006).
Bacher, N. et al. Interferon-alpha suppresses cAMP to disarm human regulatory T cells. Cancer Res. 73, 5647–5656 (2013).
Braun, D., Caramalho, I. & Demengeot, J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int. Immunol. 14, 411–419 (2002).
Le Bon, A. et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by Type I IFN. J. Immunol. 176, 2074–2078 (2006).
Swanson, C. L. et al. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J. Exp. Med. 207, 1485–1500 (2010).
Bui, J. D., Carayannopoulos, L. N., Lanier, L. L., Yokoyama, W. M. & Schreiber, R. D. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J. Immunol. 176, 905–913 (2006).
Yadav, D., Ngolab, J., Lim, R. S., Krishnamurthy, S. & Bui, J. D. Cutting edge: down-regulation of MHC class I-related chain A on tumor cells by IFN-gamma-induced microRNA. J. Immunol. 182, 39–43 (2009).
Piontek, G. E. et al. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J. Immunol. 135, 4281–4288 (1985).
Yang, I. et al. Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J. Neurosurg. 100, 310–319 (2004).
Biron, C. A., Sonnenfeld, G. & Welsh, R. M. Interferon induces natural killer cell blastogenesis in vivo. J. Leukoc. Biol. 35, 31–37 (1984).
Marrack, P., Kappler, J. & Mitchell, T. Type I interferons keep activated T cells alive. J. Exp. Med. 189, 521–530 (1999).
Brinkmann, V., Geiger, T., Alkan, S. & Heusser, C. H. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J. Exp. Med. 178, 1655–1663 (1993).
Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Jereb, B., Us-Krasovec, M., Cervek, J. & Soos, E. Intrapleural application of human leukocyte interferon (HLI) in breast cancer patients with ipsilateral pleural carcinomatosis. J. Interferon Res. 7, 357–363 (1987).
Muss, H. B. et al. Recombinant gamma interferon in advanced breast cancer: a phase II trial. Invest. New Drugs 4, 377–381 (1986).
Barreras, L., Vogel, C. L., Koch, G. & Marcus, S. G. Phase II trial of recombinant beta (IFN-betaser) interferon in the treatment of metastatic breast cancer. Invest. New Drugs 6, 211–215 (1988).
Amoroso, D. et al. Megestrol acetate plus alpha 2a interferon as second line therapy for postmenopausal patients with advanced breast cancer: results of a multicentric phase II trial. Breast Cancer Res. Treat. 33, 265–268 (1995).
Fentiman, I. S., Balkwill, F. R., Cuzick, J., Hayward, J. L. & Rubens, R. D. A trial of human alpha interferon as an adjuvant agent in breast cancer after loco-regional recurrence. Eur. J. Surg. Oncol. 13, 425–428 (1987).
Buzdar, A. U. et al. Adjuvant therapy with escalating doses of doxorubicin and cyclophosphamide with or without leukocyte alpha-interferon for stage II or III breast cancer. J. Clin. Oncol. 10, 1540–1546 (1992).
Acknowledgements
This work was supported by grant funding from the Cancer Council Victoria (B.S.P. and P.J.H.), the National Health and Medical Research Council of Australia (NHMRC) (B.S.P. and P.J.H., GNT1047747), Prostate Cancer Foundation Australia (B.S.P.), fellowship support from NHMRC and ARC (B.S.P., ARC FT130100671; P.J.H., NHMRC GNT1027020) and the Victorian Government's Operational Infrastructure Support Program. The authors are grateful to R. Smith for assistance with editing this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Glossary
- Pattern recognition receptor
-
(PRR). Member of a family of germline-encoded receptors expressed by most cells in the body that are capable of sensing pathogens and aberrant 'self' molecules to initiate inflammatory signalling cascades.
- Plasmacytoid dendritic cells
-
(pDCs). A morphologically distinct population of dendritic cells found in the circulation and lymphoid organs that are particularly high producers of type I interferon (IFN) (predominantly IFNα) in response to pattern recognition receptor stimulation.
- Danger-associated molecular patterns
-
(DAMPs). Refers to both pathogen-associated molecular patterns (PAMPs) and host-derived molecules released from injured or dying cells that activate pattern recognition receptors and the ensuing immune responses.
- poly(I:C)
-
A synthetic double-stranded RNA mimetic. Poly(I:C) triggers pattern recognition receptor activation and is used to mimic viral RNA.
- Regulatory T (Treg) cells
-
A population of CD4+ T cells that restrain the activity of effector T cells to prevent immune-mediated pathology, but also promote cancer by suppressing antitumour immunity.
- Myeloid-derived suppressor cells
-
(MDSCs). A heterogeneous population of immature myeloid cells that expand in number in cancer and settings of chronic inflammation, and have several pro-tumour functions including suppression of the immune response.
- Immune checkpoints
-
A diverse array of inhibitory pathways that maintain self-tolerance and appropriate immune function. However, therapeutic blockade of these pathways has recently proved highly effective in promoting tumour immunity.
Rights and permissions
About this article
Cite this article
Parker, B., Rautela, J. & Hertzog, P. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer 16, 131–144 (2016). https://doi.org/10.1038/nrc.2016.14
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.14
This article is cited by
-
Recent advances of engineered oncolytic viruses-based combination therapy for liver cancer
Journal of Translational Medicine (2024)
-
Single-Cell Transcription Mapping of Murine and Human Mammary Organoids Responses to Female Hormones
Journal of Mammary Gland Biology and Neoplasia (2024)
-
A novel safer CD19CAR with shRNA interference of IFN-γ can reduce multiple cytokine levels without significantly compromising its killing efficacy
Apoptosis (2024)
-
TRAF3 suppression encourages B cell recruitment and prolongs survival of microbiome-intact mice with ovarian cancer
Journal of Experimental & Clinical Cancer Research (2023)
-
The diagnostic/prognostic roles and biological function of the IFIT family members in acute myeloid leukemia
BMC Medical Genomics (2023)