Abstract
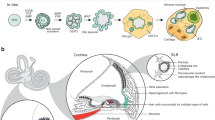
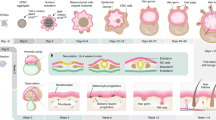
This protocol describes a culture system in which inner-ear sensory tissue is produced from mouse embryonic stem (ES) cells under chemically defined conditions. This model is amenable to basic and translational investigations into inner ear biology and regeneration. In this protocol, mouse ES cells are aggregated in 96-well plates in medium containing extracellular matrix proteins to promote epithelialization. During the first 14 d, a series of precisely timed protein and small-molecule treatments sequentially induce epithelia that represent the mouse embryonic non-neural ectoderm, preplacodal ectoderm and otic vesicle epithelia. Ultimately, these tissues develop into cysts with a pseudostratified epithelium containing inner ear hair cells and supporting cells after 16–20 d. Concurrently, sensory-like neurons generate synapse-like structures with the derived hair cells. We have designated the stem cell–derived epithelia harboring hair cells, supporting cells and sensory-like neurons as inner ear organoids. This method provides a reproducible and scalable means to generate inner ear sensory tissue in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 493, 318–326 (2013).
Sasai, Y., Eiraku, M. & Suga, H. In vitro organogenesis in three dimensions: self-organising stem cells. Development 139, 4111–4121 (2012).
Koehler, K.R., Mikosz, A.M., Molosh, A.I., Patel, D. & Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013).
Gonzalez-Cordero, A. et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 31, 741–747 (2013).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Eiraku, M. & Sasai, Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat. Protoc. 7, 69–79 (2012).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Suga, H. et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480, 57–62 (2011).
Nasu, M. et al. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS ONE 7, e53024 (2012).
Lancaster, M. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Spence, J.R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2010).
McCracken, K.W., Howell, J.C., Wells, J.M. & Spence, J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928 (2011).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Li, L. et al. Location of transient ectodermal progenitor potential in mouse development. Development 140, 4533–4543 (2013).
Cajal, M. et al. Clonal and molecular analysis of the prospective anterior neural boundary in the mouse embryo. Development 139, 423–436 (2012).
Beddington, S.P. An autoradiographic analysis of the potency of embryonic ectoderm in the 8th day postimplantation mouse embryo. J. Embryol. Exp. Morphol. 64, 87–104 (1981).
Beddington, R.S. An autoradiographic analysis of tissue potency in different regions of the embryonic ectoderm during gastrulation in the mouse. J. Embryol Exp. Morphol. 69, 265–285 (1982).
Wilson, P.A. & Hemmati-Brivanlou, A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376, 331–333 (1995).
Wilson, P.A., Lagna, G., Suzuki, A. & Hemmati-Brivanlou, A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development 124, 3177–3184 (1997).
Kwon, H.-J., Bhat, N., Sweet, E.M., Cornell, R.A. & Riley, B.B. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 6, e1001133 (2010).
Barth, K.A. et al. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development 126, 4977–4987 (1999).
Neave, B., Holder, N. & Patient, R. A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech. Dev. 62, 183–195 (1997).
Harvey, N.T. et al. Response to BMP4 signalling during ES cell differentiation defines intermediates of the ectoderm lineage. J. Cell Sci. 123, 1796–1804 (2010).
Pieper, M., Ahrens, K., Rink, E., Peter, A. & Schlosser, G. Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development 139, 1175–1187 (2012).
Reichert, S., Randall, R.A. & Hill, C.S. A BMP regulatory network controls ectodermal cell fate decisions at the neural plate border. Development 140, 4435–4444 (2013).
Grocott, T., Tambalo, M. & Streit, A. The peripheral sensory nervous system in the vertebrate head: a gene regulatory perspective. Dev. Biol. 370, 3–23 (2012).
Ahrens, K. & Schlosser, G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev. Biol. 288, 40–59 (2005).
Brugmann, S.A., Pandur, P.D., Kenyon, K.L., Pignoni, F. & Moody, S.A. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development 131, 5871–5881 (2004).
Litsiou, A., Hanson, S. & Streit, A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development 132, 4051–4062 (2005).
Schlosser, G. Induction and specification of cranial placodes. Dev. Biol. 294, 303–351 (2006).
Patthey, C. & Gunhaga, L. Specification and regionalisation of the neural plate border. Eur. J. Neurosci. 34, 1516–1528 (2011).
Bhattacharyya, S. & Bronner-Fraser, M. Competence, specification and commitment to an olfactory placode fate. Development 135, 4165–4177 (2008).
Baker, C.V., Stark, M.R., Marcelle, C. & Bronner-Fraser, M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development 126, 147–156 (1999).
Ladher, R.K., O'Neill, P. & Begbie, J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development 137, 1777–1785 (2010).
Wright, T.J. & Mansour, S.L. Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130, 3379–3390 (2003).
Urness, L.D., Paxton, C.N., Wang, X., Schoenwolf, G.C. & Mansour, S.L. FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev. Biol. 340, 595–604 (2010).
Ohyama, T., Mohamed, O.A., Taketo, M.M., Dufort, D. & Groves, A.K. Wnt signals mediate a fate decision between otic placode and epidermis. Development 133, 865–875 (2006).
Riccomagno, M.M., Takada, S. & Epstein, D.J. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 19, 1612–1623 (2005).
Freter, S., Muta, Y., Mak, S.-S., Rinkwitz, S. & Ladher, R.K. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development 135, 3415–3424 (2008).
Groves, A.K. & Bronner-Fraser, M. Competence, specification and commitment in otic placode induction. Development 127, 3489–3499 (2000).
Groves, A.K. & Fekete, D.M. Shaping sound in space: the regulation of inner ear patterning. Development 139, 245–257 (2011).
Neves, J., Parada, C., Chamizo, M. & Giraldez, F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development 138, 735–744 (2011).
Appler, J.M. & Goodrich, L.V. Connecting the ear to the brain: molecular mechanisms of auditory circuit assembly. Prog. Neurobiol. 93, 488–508 (2011).
Laine, H., Sulg, M., Kirjavainen, A. & Pirvola, U. Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev. Biol. 337, 134–146 (2010).
Oesterle, E.C., Campbell, S., Taylor, R.R., Forge, A. & Hume, C.R. Sox2 and Jagged1 expression in normal and drug-damaged adult mouse inner ear. J. Assoc. Res. Otolaryngol. 9, 65–89 (2007).
Xiang, M., Gao, W.Q., Hasson, T. & Shin, J.J. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development 125, 3935–3946 (1998).
Watanabe, K. et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288–296 (2005).
Muñoz-Sanjuán, I. & Brivanlou, A.H. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3, 271–280 (2002).
James, D., Levine, A.J., Besser, D. & Hemmati-Brivanlou, A. TGF-β/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132, 1273–1282 (2005).
Camus, A., Perea-Gomez, A., Moreau, A. & Collignon, J. Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev. Biol. 295, 743–755 (2006).
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Muguruma, K. et al. Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat. Neurosci. 13, 1171–1180 (2010).
Bouchard, M., de Caprona, D., Busslinger, M., Xu, P. & Fritzsch, B. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev. Biol. 10, 89 (2010).
Christophorou, N.A.D., Mende, M., Lleras-Forero, L., Grocott, T. & Streit, A. Pax2 coordinates epithelial morphogenesis and cell fate in the inner ear. Dev. Biol. 345, 180–190 (2010).
Warchol, M.E. & Richardson, G.P. Expression of the Pax2 transcription factor is associated with vestibular phenotype in the avian inner ear. Dev. Neurobiol. 69, 191–202 (2009).
Lysakowski, A. et al. Molecular microdomains in a sensory terminal, the vestibular calyx ending. J. Neurosci. 31, 10101–10114 (2011).
Li, A., Xue, J. & Peterson, E.H. Architecture of the mouse utricle: macular organization and hair bundle heights. J. Neurophysiol. 99, 718–733 (2008).
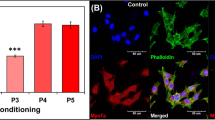
Li, H., Roblin, G., Liu, H. & Heller, S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA 100, 13495–13500 (2003).
Oshima, K. et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141, 704–716 (2010).
Ouji, Y., Ishizaka, S., Nakamura-Uchiyama, F. & Yoshikawa, M. In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium. Cell Death Dis. 3, e314 (2012).
Chen, W. et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 490, 278–282 (2012).
Ouji, Y., Ishizaka, S., Nakamura-Uchiyama, F., Wanaka, A. & Yoshikawa, M. Induction of inner ear hair cell-like cells from Math1-transfected mouse ES cells. Cell Death Dis. 4, e700 (2013).
Brigande, J.V. & Heller, S. Quo vadis, hair cell regeneration? Nat. Neurosci. 12, 679–685 (2009).
Ding, Q. et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 12, 238–251 (2013).
Ding, Q. et al. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell 12, 393–394 (2013).
Trowe, M.-O. et al. Loss of Sox9 in the periotic mesenchyme affects mesenchymal expansion and differentiation, and epithelial morphogenesis during cochlea development in the mouse. Dev. Biol. 342, 51–62 (2010).
Ying, Q.-L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Gaboyard, S. et al. Three-dimensional culture of newborn rat utricle using an extracellular matrix promotes formation of a cyst. Neuroscience 133, 253–265 (2005).
Lerner, S.A., Matz, G.J. & Hawkins, J.E. Aminoglycoside Ototoxicity (Little Brown and Company, 1981).
Yokomizo, T. et al. Whole-mount three-dimensional imaging of internally localized immunostained cells within mouse embryos. Nat. Protoc. 7, 421–431 (2012).
Acknowledgements
We thank A. Mikosz, D. Patel and S. Majumdar for their technical assistance and comments on the manuscript. This work was supported by US National Institutes of Health (NIH) grants RC1DC010706, R21DC012617, and R01DC013294 and an Indiana University School of Medicine Biomedical Research Grant (to E.H.). K.R.K. was supported by a Paul and Carole Stark Neurosciences Fellowship and an Indiana Clinical and Translational Science Institute Predoctoral Fellowship (NIH TL1RR025759).
Author information
Authors and Affiliations
Contributions
K.R.K. designed the protocol, made figures and wrote the manuscript. E.H. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.R.K. and E.H. have disclosed these findings to the Indiana University Research and Technology Corporation, which has filed a patent to ensure broad use of the methods for studying inner-ear development and disease mechanisms. All protocols and methods remain freely available for academic and nonprofit research in perpetuity.
Integrated supplementary information
Supplementary Figure 1 Aggregate imaging chamber.
a, Typical imaging chamber constructed from two cover glasses and a silicone isolator. One aggregate (sample) is loaded in the chamber. Note the air bubble that forms during the loading process. b, Projection drawing of an aggregate imaging chamber. Note that this chamber design is an example and can be easily customized using different sized cover glasses or silicone isolators.
Supplementary information
Supplementary Figure 1
Aggregate imaging chamber. (PDF 1922 kb)
Rights and permissions
About this article
Cite this article
Koehler, K., Hashino, E. 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat Protoc 9, 1229–1244 (2014). https://doi.org/10.1038/nprot.2014.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.100
This article is cited by
-
Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear
Neuroscience Bulletin (2024)
-
MLL3/MLL4 methyltransferase activities control early embryonic development and embryonic stem cell differentiation in a lineage-selective manner
Nature Genetics (2023)
-
CHD7 regulates otic lineage specification and hair cell differentiation in human inner ear organoids
Nature Communications (2022)
-
Differentiation of embryonic stem cells into a putative hair cell-progenitor cells via co-culture with HEI-OC1 cells
Scientific Reports (2021)
-
Photobiomodulation with a wavelength > 800 nm induces morphological changes in stem cells within otic organoids and scala media of the cochlea
Lasers in Medical Science (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.