Abstract
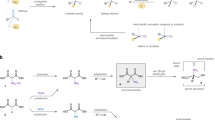
This protocol details the most commonly used nuclear magnetic resonance (NMR)-based method for deducing the configuration of otherwise unknown stereogenic, secondary carbinol (alcohol) centers (R1R2CHOH (or the analogous amines where OH is replaced by NH2)). This 'Mosher ester analysis' relies on the fact that the protons in diastereomeric α-methoxy-α-trifluoromethylphenylacetic acid (MTPA) esters (i.e., those derived from conjugation of the carbinol under interrogation with MTPA) display different arrays of chemical shifts (δs) in their 1H NMR spectra. The protocol consists of the following: (i) preparation of each of the diastereomeric S- and R-MTPA esters and (ii) comparative (ΔδSR) analysis of the 1H NMR spectral data of these two esters. By analyzing the sign of the difference in chemical shifts for a number of analogous pairs of protons (the set of ΔδSR values) in the diastereomeric esters (or amides), the absolute configuration of the original carbinol (or amino) stereocenter can be reliably deduced. A typical Mosher ester analysis requires approximately 4–6 h of active effort over a 1- to 2-d period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stephens, T.D. & Brynner, R. Dark Remedy: The Impact of Thalidomide and Its Revival as a Vital Medicine (Perseus Publishing, Cambridge, MA, 2001).
Seco, J.M., Quiñoá, E. & Riguera, R. The assignment of absolute configuration by NMR. Chem. Rev. 104, 17–117 (2004).
Dale, J.A. & Mosher, H.S. Nuclear magnetic resonance enantiomer reagents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 95, 512–519 (1973).
Hoye, T.R. & Renner, M.K. MTPA (Mosher) amides of cyclic secondary amines: conformational aspects and a useful method for assignment of amine configuration. J. Org. Chem. 61, 2056–2064 (1996).
Dale, J.A., Dull, D.L. & Mosher, H.S. α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J. Org. Chem. 34, 2543–2549 (1969).
Ward, D.E. & Rhee, C.K. A simple method for the microscale preparation of Mosher's acid chloride. Tetrahedron Lett. 32, 7165–7166 (1991).
Sullivan, G.R., Dale, J.A. & Mosher, H.S. Correlation of configuration and 19F chemical shifts of α-methoxy-α-trifluoromethylphenylacetate derivatives. J. Org. Chem. 38, 2143–2147 (1973).
Ohtani, I., Kusumi, T., Ishitsuka, M.O. & Kakisawa, H. Absolute configurations of marine diterpenes possessing a xenicane skeleton. An application of an advanced Mosher's method. Tetrahedron Lett. 30, 3147–3150 (1989).
Ohtani, I., Kusumi, T., Kashman, Y. & Kakisawa, H. A new aspect of the high-field NMR application of Mosher's method. The absolute configuration of marine triterpene sipholenol A. J. Org. Chem. 56, 1296–1298 (1991).
Ohtani, I., Kusumi, T., Kashman, Y. & Kakisawa, H. High-field FT NMR application of Mosher's method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 113, 4092–4096 (1991).
Parker, D. NMR determination of enantiomeric purity. Chem. Rev. 91, 1441–1457 (1991).
Trost, B.M. et al. On the use of the O-methylmandelate ester for establishment of absolute configuration of secondary alcohols. J. Org. Chem. 51, 2370–2374 (1986).
Moon, S. et al. (+)-7S-Hydroxyxestospongin A from the marine sponge Xestospongia sp. and absolute configuration of (+)-xestospongin D. J. Nat. Prod. 65, 249–254 (2002).
Still, W.C., Kahn, M. & Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 43, 2923–2925 (1978).
Cahn, R.S., Ingold, C.K. & Prelog, V. The specification of asymmetric configuration in organic chemistry. Experientia 12, 81–94 (1956).
Joshi, B.S. & Pelletier, S.W. A cautionary note on the use of commercial (R)-MTPA-Cl and (S)-MTPA-Cl in determination of absolute configuration by Mosher ester analysis. Heterocycles 51, 183–184 (1999).
Garo, E. et al. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens. J. Nat. Prod. 66, 423–426 (2003).
Hoye, T.R. & Jeffrey, C.S. Student empowerment through 'mini-microscale' reactions: the epoxidation of 1 mg of geraniol. J. Chem. Educ. 83, 919–920 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoye, T., Jeffrey, C. & Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat Protoc 2, 2451–2458 (2007). https://doi.org/10.1038/nprot.2007.354
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.354
This article is cited by
-
THRONCAT: metabolic labeling of newly synthesized proteins using a bioorthogonal threonine analog
Nature Communications (2023)
-
Structural and stereochemical determinants for hGAT3 inhibition: development of novel conformationally constrained and substituted analogs of (S)-isoserine
Medicinal Chemistry Research (2023)
-
A computer algorithm to discover iterative sequences of organic reactions
Nature Synthesis (2022)
-
Determination of the Absolute Configuration of the Male-Produced Sex Pheromone of the Stink Bug Pellaea stictica, (2R,4R,8R)-2,4,8,13-Tetramethyltetradecan-1-ol by Stereoselective Synthesis Coupled with Enantiomeric Resolution
Journal of Chemical Ecology (2022)
-
From Relative to Absolute Stereochemistry of Secondary Metabolites: Applications in Plant Chemistry
Revista Brasileira de Farmacognosia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.