Abstract
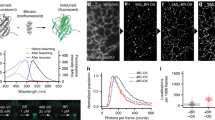
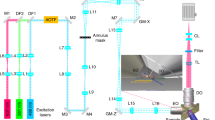
Low-light microscopy methods are receiving increased attention as new applications have emerged. One such application is to allow longitudinal imaging of light-sensitive cells with no phototoxicity and no photobleaching of fluorescent biomarkers. Another application is for imaging signals that are inherently dim and undetectable using standard microscopy techniques, such as bioluminescence, chemiluminescence or radioluminescence. In this protocol, we provide instructions on how to build a modular low-light microscope (1–4 d) by coupling two microscope objective lenses, back to back from each other, using standard optomechanical components. We also provide directions on how to image dim signals such as those of radioluminescence (1–1.5 h), bioluminescence (∼30 min) and low-excitation fluorescence (∼15 min). In particular, radioluminescence microscopy is explained in detail, as it is a newly developed technique that enables the study of small-molecule transport (e.g., radiolabeled drugs, metabolic precursors and nuclear medicine contrast agents) by single cells without perturbing endogenous biochemical processes. In this imaging technique, a scintillator crystal (e.g., CdWO4) is placed in close proximity to the radiolabeled cells, where it converts the radioactive decays into optical flashes detectable using a sensitive camera. Using the image reconstruction toolkit provided in this protocol, the flashes can be reconstructed to yield high-resolution images of the radiotracer distribution. With appropriate timing, the three aforementioned imaging modalities may be performed together on a population of live cells, allowing the user to perform parallel functional studies of cell heterogeneity at the single-cell level.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Welsh, D.K. & Noguchi, T. Cellular bioluminescence imaging. Cold Spring Harb. Protoc. 2012, 852–866
Badr, C.E. & Tannous, B.A. Bioluminescence imaging: progress and applications. Trends Biotechnol. 29, 624–633 (2011).
Coulon, V. et al. Subcellular imaging of dynamic protein interactions by bioluminescence resonance energy transfer. Biophys. J. 94, 1001–1009 (2008).
Luker, K.E. et al. Kinetics of regulated protein–protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc. Natl. Acad. Sci. USA 101, 12288–12293 (2004).
Shimomura, O. & Johnson, F.H. Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence. Nature 227, 1356–1357 (1970).
Welsh, D.K., Yoo, S.-H., Liu, A.C., Takahashi, J.S. & Kay, S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 14, 2289–2295 (2004).
Gambhir, S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2, 683–693 (2002).
Weber, M.J. Inorganic scintillators: today and tomorrow. J. Lumin. 100, 35–45 (2002).
Pratx, G. et al. Radioluminescence microscopy: measuring the heterogeneous uptake of radiotracers in single living cells. PloS One 7, e46285 (2012).
Sengupta, D. & Pratx, G. Single-cell characterization of 18F-FLT uptake with radioluminescence microscopy. J. Nucl. Med. 57, 1136–1140 (2016).
Natarajan, A., Türkcan, S., Gambhir, S.S. & Pratx, G. Multiscale framework for imaging radiolabeled therapeutics. Mol. Pharm. 12, 4554–4560 (2015).
Puncher, M.R. & Blower, P.J. Radionuclide targeting and dosimetry at the microscopic level: the role of microautoradiography. Eur. J. Nucl. Med. 21, 1347–1365 (1994).
Miller, B.W. et al. The iQID camera: an ionizing-radiation quantum imaging detector. Nucl. Instr. Meth. Phys. Res. A 767, 146–152 (2014).
Barthe, N., Chatti, K., Coulon, P., Mantrejean, S. & Basse-Cathalinat, B. Recent technologic developments on high-resolution beta imaging systems for quantitative autoradiography and double labeling applications. Nucl. Instr. Meth. Phys. Res. A 527, 41–45 (2004).
Ogoh, K. et al. Bioluminescence microscopy using a short focallength imaging lens. J. Microsc. 253, 191–197 (2014).
Goda, K. et al. Combining fluorescence and bioluminescence microscopy. Microsc. Res. Tech. 78, 715–722 (2015).
Hoshino, H., Nakajima, Y. & Ohmiya, Y. Luciferase-YFP fusion tag with enhanced emission for single-cell luminescence imaging. Nat. Methods 4, 637–639 (2007).
Kim, T.J., Tuerkcan, S., Ceballos, A. & Pratx, G. Modular platform for low-light microscopy. Biomed. Opt. Express 6, 4585–4598 (2015).
Türkcan, S. et al. Single-cell analysis of [18F] fluorodeoxyglucose uptake by droplet radiofluidics. Anal. Chem. 87, 6667–6673 (2015).
Nakatsu, T. et al. Structural basis for the spectral difference in luciferase bioluminescence. Nature 440, 372–376 (2006).
Greer, L.F. & Szalay, A.A. Imaging of light emission from the expression of luciferases in living cells and organisms: a review. Luminescence 17, 43–74 (2002).
Contag, C.H. & Bachmann, M.H. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 4, 235–260 (2002).
Lambrechts, D. et al. A causal relation between bioluminescence and oxygen to quantify the cell niche. PLoS One 9, e97572 (2014).
Pratx, G. et al. High-resolution radioluminescence microscopy of 18F-FDG uptake by reconstructing the β-ionization track. J. Nucl. Med. 54, 1841–1846 (2013).
Sengupta, D. et al. Bright Lu2O3: Eu thin-film scintillators for high-resolution radioluminescence microscopy. Adv. Healthc. Mater. 4, 2064–2070 (2015).
Spitzer, M., Wildenhain, J., Rappsilber, J. & Tyers, M. BoxPlotR: a web tool for generation of box plots. Nat. Methods 11, 121–122 (2014).
Acknowledgements
This work was supported by funding from the National Institutes of Health (NIH) under grants 5R01CA186275 and 1R21CA193001. T.J.K. was supported in part by NCI training grant T32 CA118681. The authors gratefully acknowledge the support of Olympus for its loan of the LV200 microscope.
Author information
Authors and Affiliations
Contributions
T.J.K. designed and built the low-light microscope system; T.J.K. and G.P. developed the graphical user interface version of ORBIT; T.J.K. and S.T. prepared radiolabeled samples; T.J.K. performed experiments and analyzed data; T.J.K. and G.P. prepared the manuscript; S.T. provided manuscript feedback; and G.P. provided guidance for the entire project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Schematic diagram of Köhler illumination.
The green beam path represents the excitation path and the red represents the emission path.
Supplementary Figure 2 Microscope base components and assembly process.
Numbers correspond to assembly flow as indicated in Steps 1 – 3.
Supplementary Figure 3 Camera mount components and assembly process.
Numbers correspond to assembly flow as indicated in Steps 4 – 7.
Supplementary Figure 4 Spirit level alignment.
Example of using (a) bullseye spirit level [Step 8] and (b) tubular spirit level [Step 11] to align components.
Supplementary Figure 5 Tube lens mount components and assembly process.
Numbers correspond to assembly flow as indicated in Step 9A.
Supplementary Figure 6 Dichroic cube mount components and assembly process for epifluorescence imaging feature.
Numbers correspond to assembly flow as indicated in Step 9B.
Supplementary Figure 7 Motorized microscope objective stage components and assembly process.
Numbers correspond to assembly flow as indicated in Steps 10 – 16.
Supplementary Figure 8 Microscope objective mounted on translation stage adapter.
The base of the objective lens (above the treaded section) should be resting on the tube lens mount. Once the objective lens is properly secured to the stage mount, shift the lens mount up and down to ensure that the lens threading does not rub against the mount.
Supplementary Figure 9 Microscope specimen stage components and assembly process.
Numbers correspond to assembly flow as indicated in Steps 17 – 26.
Supplementary Figure 10 Fabrication steps for microscope stage.
(a) Two pairs of 1” x 3” glass slides are bonded to each other by applying superglue on the areas shown by blue arrows. Apply enough superglue so that the entire area of the base is covered. (b) Bond two additional glass slides on top and bottom of the two slide pairs and (c) wait until the glue has cured. (d) Mount the completed glass stage on the XY translation adapter, and tightly position it by sliding and locking the slider. The glass stage can then be secured by tightening four screws on the top of the XY translation adapter.
Supplementary Figure 11 Transillumination source components and assembly process.
Numbers correspond to assembly flow as indicated in Step 27A.
Supplementary Figure 12 Epifluorescence illumination module components and assembly process.
Numbers correspond to assembly flow as indicated in Step 27B.
Supplementary Figure 13 Schematic diagram showing lens and diaphragm location for Köhler illumination.
Beam path showing the approximate location of lenses and iris diaphragms, where f is the focal length and fb is the back focal length of the lens. The actual positions may vary during the setup; hence calibration must be performed to achieve good Köhler illumination. For example, the first iris diaphragm (condenser diaphragm) must be located where the LED source is clearly visible after the condenser lens.
Supplementary Figure 14 Calibration of microscope magnifying power.
To achieve the desired magnifying power, (a) acquire an image of a calibration grid and measure the grid length (in pixels) with ImageJ. The effective magnification power is given by MLLM =detector pixel size [μm/px] /(grid size [μm] /measured number of pixels [px]). (b) If needed, adjust camera position. Repeat steps if necessary.
Supplementary Figure 15 Imaging area comparison with and without epifluorescence illumination add-on.
(a) Micrographs of 100 μm spaced target grid captured with and epi-fluorescence illumination module. Significant vignetting can be observed. (b) In comparison, the imaging area is larger if the epi-fluorescence module is omitted. Scale bar, 100 μm.
Supplementary Figure 16 Comparison between analog and digital scintillation images.
Analog scintillation images captured with (a) no binning, 1200× EM gain, and 1 min exposure time and (b) 4 binning, ~700× EM gain, and 2 min exposure time. (c) Digitial scintillation image captured with 4 binning, 1200× EM gain, 30 ms exposure time per frame, and 20,000 frames. Hot spots (red arrows) are caused by stray ionizing radiation directly hitting the EMCCD detector, frequently appears in analog images, whereas such signals are rejected in digitally reconstructed images. Scale bar, 200 μm.
Supplementary Figure 17 Multimodal micrographs of confluent MDA-MB-231 cells.
Brightfield, fluorescence, bioluminescence, and overlay images from MDA-MB-231/Luc cells. Top row. Nikon 20x objective lens (effective magnification of 4x). Scale bar, 200 μm. Bottom row. Olympus 40x oil-immersion objective lens (effective magnification of ~11x). Scale bar, 100 μm.
Supplementary Figure 18 Example of bioluminescence imaging captured under short exposure times for real-time imaging.
(a) Brightfield image of MDA-MB-231/Luc cells and its corresponding bioluminescence images captured with different imaging parameters: (b) no binning, ~1060× EM gain, and 20 s exposure time; (c) no binning, 1200× EM gain, and 3 s exposure time; (d) 2 binning, 1200× EM gain, and 0.75 s exposure time. Scale bar, 200 μm.
Supplementary information
Combo pdf
Supplementary Figures 1–18, Supplementary Table 1 and the Supplementary Note. (PDF 2246 kb)
Rights and permissions
About this article
Cite this article
Kim, T., Türkcan, S. & Pratx, G. Modular low-light microscope for imaging cellular bioluminescence and radioluminescence. Nat Protoc 12, 1055–1076 (2017). https://doi.org/10.1038/nprot.2017.008
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.008
This article is cited by
-
High-resolution positron emission microscopy of patient-derived tumor organoids
Nature Communications (2021)
-
High-resolution radioluminescence microscopy of FDG uptake in an engineered 3D tumor-stoma model
European Journal of Nuclear Medicine and Molecular Imaging (2021)
-
Whole-body tracking of single cells via positron emission tomography
Nature Biomedical Engineering (2020)
-
Dependence of fluorodeoxyglucose (FDG) uptake on cell cycle and dry mass: a single-cell study using a multi-modal radiography platform
Scientific Reports (2020)
-
Thermochemiluminescent peroxide crystals
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.