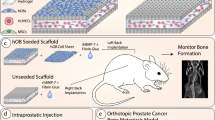
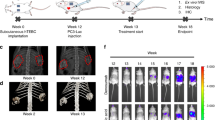
Abstract
Current in vivo models for investigating human primary bone tumors and cancer metastasis to the bone rely on the injection of human cancer cells into the mouse skeleton. This approach does not mimic species-specific mechanisms occurring in human diseases and may preclude successful clinical translation. We have developed a protocol to engineer humanized bone within immunodeficient hosts, which can be adapted to study the interactions between human cancer cells and a humanized bone microenvironment in vivo. A researcher trained in the principles of tissue engineering will be able to execute the protocol and yield study results within 4–6 months. Additive biomanufactured scaffolds seeded and cultured with human bone-forming cells are implanted ectopically in combination with osteogenic factors into mice to generate a physiological bone 'organ', which is partially humanized. The model comprises human bone cells and secreted extracellular matrix (ECM); however, other components of the engineered tissue, such as the vasculature, are of murine origin. The model can be further humanized through the engraftment of human hematopoietic stem cells (HSCs) that can lead to human hematopoiesis within the murine host. The humanized organ bone model has been well characterized and validated and allows dissection of some of the mechanisms of the bone metastatic processes in prostate and breast cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12, 6243s–6249s (2006).
Taubenberger, A.V. In vitro microenvironments to study breast cancer bone colonisation. Adv. Drug Deliv. Rev. 79–80, 135–144 (2014).
Villasante, A., Marturano-Kruik, A. & Vunjak-Novakovic, G. Bioengineered human tumor within a bone niche. Biomaterials 35, 5785–5794 (2014).
Tan, P.H., Aung, K.Z., Toh, S.L., Goh, J.C. & Nathan, S.S. Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology. Biomaterials 32, 6131–6137 (2011).
Hamdi, D.H. et al. In vitro engineering of human 3D chondrosarcoma: a preclinical model relevant for investigations of radiation quality impact. BMC Cancer 15, 579 (2015).
Khanna, C. & Hunter, K. Modeling metastasis in vivo. Carcinogenesis 26, 513–523 (2005).
Gupta, P.B. & Kuperwasser, C. Disease models of breast cancer. Drug Discov. Today Dis. Models 1, 9–16 (2004).
Ek, E.T., Dass, C.R. & Choong, P.F. Commonly used mouse models of osteosarcoma. Crit. Rev. Oncol. Hematol. 60, 1–8 (2006).
Janeway, K.A. & Walkley, C.R. Modeling human osteosarcoma in the mouse: from bedside to bench. Bone 47, 859–865 (2010).
Kretschmann, K.L. & Welm, A.L. Mouse models of breast cancer metastasis to bone. Cancer Metastasis Rev. 31, 579–583 (2012).
Singh, A.S. & Figg, W.D. In vivo models of prostate cancer metastasis to bone. J. Urol. 174, 820–826 (2005).
Tolcher, A.W. et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J. Clin. Oncol. 17, 478–484 (1999).
Kostenuik, P.J. et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 24, 182–195 (2009).
Shtivelman, E. & Namikawa, R. Species-specific metastasis of human tumor cells in the severe combined immunodeficiency mouse engrafted with human tissue. Proc. Natl. Acad. Sci. USA 92, 4661–4665 (1995).
Nemeth, J.A. et al. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 59, 1987–1993 (1999).
Kuperwasser, C. et al. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 65, 6130–6138 (2005).
Hesami, P. et al. A humanized tissue-engineered in vivo model to dissect interactions between human prostate cancer cells and human bone. Clin. Exp. Metastasis 31, 435–446 (2014).
Holzapfel, B.M. et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35, 4108–4115 (2014).
Thibaudeau, L. et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis. Model. Mech. 7, 299–309 (2014).
Quent, V.M.C., Theodoropoulos, C., Hutmacher, D.W. & Reichert, J.C. Differential osteogenicity of multiple donor-derived human mesenchymal stem cells and osteoblasts in monolayer, scaffold-based 3D culture and in vivo. Biomed. Tech. 61, 253–266 (2016).
Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 21, 2529–2543 (2000).
Hutmacher, D.W., Schantz, J.T., Lam, C.X., Tan, K.C. & Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 1, 245–260 (2007).
Hutmacher, D.W. & Cool, S. Concepts of scaffold-based tissue engineering--the rationale to use solid free-form fabrication techniques. J. Cell. Mol. Med. 11, 654–669 (2007).
Reichert, J.C. et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 4, 141ra193 (2012).
Zhou, Y. et al. Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials 28, 814–824 (2007).
Brown, T.D. et al. Design and fabrication of tubular scaffolds via direct writing in a melt electrospinning mode. Biointerphases 7, 13 (2012).
Kolambkar, Y.M. et al. Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone 49, 485–492 (2011).
Hutmacher, D.W. & Dalton, P.D. Melt electrospinning. Chem. Asian J. 6, 44–56 (2011).
Vaquette, C. & Cooper-White, J.J. Increasing electrospun scaffold pore size with tailored collectors for improved cell penetration. Acta Biomater. 7, 2544–2557 (2011).
Brown, T.D., Dalton, P.D. & Hutmacher, D.W. Direct writing by way of melt electrospinning. Adv. Mater. 23, 5651–5657 (2011).
Farrugia, B.L. et al. Dermal fibroblast infiltration of poly(e-caprolactone) scaffolds fabricated by melt electrospinning in a direct writing mode. Biofabrication 5, 025001 (2013).
Vaquette, C., Ivanovski, S., Hamlet, S.M. & Hutmacher, D.W. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 34, 5538–5551 (2013).
Chatterjea, A., Meijer, G., van Blitterswijk, C. & de Boer, J. Clinical application of human mesenchymal stromal cells for bone tissue engineering. Stem Cells Int. 2010, 215625 (2010).
Kern, S., Eichler, H., Stoeve, J., Kluter, H. & Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24, 1294–1301 (2006).
Reinisch, A. et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood 125, 249–260 (2015).
Reichert, J.C., Quent, V.M., Noth, U. & Hutmacher, D.W. Ovine cortical osteoblasts outperform bone marrow cells in an ectopic bone assay. J. Tissue Eng. Regen. Med. 5, 831–844 (2011).
Dickhut, A. et al. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J. Cell. Physiol. 219, 219–226 (2009).
Zhang, Z.Y. et al. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells 27, 126–137 (2009).
Holzapfel, B.M. et al. Tissue engineered humanized bone supports human hematopoiesis in vivo. Biomaterials 61, 103–114 (2015).
Ooi, L.L., Zheng, Y., Stalgis-Bilinski, K. & Dunstan, C.R. The bone remodeling environment is a factor in breast cancer bone metastasis. Bone 48, 66–70 (2011).
Mastro, A.M., Gay, C.V. & Welch, D.R. The skeleton as a unique environment for breast cancer cells. Clin. Exp. Metastasis 20, 275–284 (2003).
Schneider, J.G., Amend, S.R. & Weilbaecher, K.N. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone 48, 54–65 (2011).
Patel, L.R., Camacho, D.F., Shiozawa, Y., Pienta, K.J. & Taichman, R.S. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 7, 1285–1297 (2011).
Moreau, J.E. et al. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 67, 10304–10308 (2007).
Taichman, R.S. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 105, 2631–2639 (2005).
Kaplan, R.N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Shiozawa, Y. et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Invest. 121, 1298–1312 (2011).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Muerza-Cascante, M.L., Haylock, D., Hutmacher, D.W. & Dalton, P.D. Melt electrospinning and its technologization in tissue engineering. Tissue Eng. Part B Rev. 21, 187–202 (2015).
Song, J. et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood 115, 2592–2600 (2010).
Yoshino, H. et al. Natural killer cell depletion by anti-asialo GM1 antiserum treatment enhances human hematopoietic stem cell engraftment in NOD/Shi-scid mice. Bone Marrow Transplant. 26, 1211–1216 (2000).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106, 1565–1573 (2005).
Ito, R., Takahashi, T., Katano, I. & Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 9, 208–214 (2012).
Macchiarini, F., Manz, M.G., Palucka, A.K. & Shultz, L.D. Humanized mice: are we there yet? J. Exp. Med. 202, 1307–1311 (2005).
Lee, J. et al. Implantable microenvironments to attract hematopoietic stem/cancer cells. Proc. Natl. Acad. Sci. USA 109, 19638–19643 (2012).
Groen, R.W. et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood 120, e9–e16 (2012).
Chen, Y. et al. Human extramedullary bone marrow in mice: a novel in vivo model of genetically controlled hematopoietic microenvironment. Blood 119, 4971–4980 (2012).
Holzapfel, B.M., Wagner, F., Thibaudeau, L., Levesque, J.P. & Hutmacher, D.W. Concise review: humanized models of tumor immunology in the 21st century: convergence of cancer research and tissue engineering. Stem Cells 33, 1696–1704 (2015).
Thibaudeau, L. et al. Mimicking breast cancer-induced bone metastasis in vivo: current transplantation models and advanced humanized strategies. Cancer Metastasis Rev. 33, 721–735 (2014).
Bersani, F. et al. Bioengineered implantable scaffolds as a tool to study stromal-derived factors in metastatic cancer models. Cancer Res. 74, 7229–7238 (2014).
Schuster, J., Zhang, J. & Longo, M. A novel human osteoblast-derived severe combined immunodeficiency mouse model of bone metastasis. J. Neurosurg. Spine 4, 388–391 (2006).
Seib, F.P., Berry, J.E., Shiozawa, Y., Taichman, R.S. & Kaplan, D.L. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 51, 313–319 (2015).
Goldstein, R.H., Reagan, M.R., Anderson, K., Kaplan, D.L. & Rosenblatt, M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 70, 10044–10050 (2010).
Battula, V.L. et al. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood 122, 357–366 (2013).
Azarin, S.M. et al. In vivo capture and label-free detection of early metastatic cells. Nat. Commun. 6, 8094 (2015).
Dondossola, E. et al. Abstract 4941: a humanized bone model for preclinical monitoring of prostate cancer lesions by intravital multiphoton microscopy. Cancer Res. 74, 4941 (2014).
Hughes, C.S., Postovit, L.M. & Lajoie, G.A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 (2010).
Vukicevic, S. et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 202, 1–8 (1992).
Thibaudeau, L., Holzapfel, B.M. & Hutmacher, D.W. Humanized mice models for primary bone tumor and bone metastasis research. Cell Cycle 14, 2191–2192 (2015).
Holzapfel, B.M. et al. Humanised xenograft models of bone metastasis revisited: novel insights into species-specific mechanisms of cancer cell osteotropism. Cancer Metastasis Rev. 32, 129–145 (2013).
Thibaudeau, L. et al. New mechanistic insights of integrin β1 in breast cancer bone colonization. Oncotarget 6, 332–344 (2015).
Wagner, F. et al. A validated preclinical animal model for primary bone tumor research. J. Bone Joint Surg. Am. 98, 916–925 (2016).
Kuznetsov, S.A. et al. Age-dependent demise of GNAS-mutated skeletal stem cells and 'normalization' of fibrous dysplasia of bone. J. Bone Miner. Res. 23, 1731–1740 (2008).
Morrison, S.J. & Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Heike, Y., Ohira, T., Takahashi, M. & Saijo, N. Long-term human hematopoiesis in SCID-hu mice bearing transplanted fragments of adult bone and bone marrow cells. Blood 86, 524–530 (1995).
Hubin, F. et al. Maintenance of functional human cancellous bone and human hematopoiesis in NOD/SCID mice. Cell Transplant. 13, 823–831 (2004).
Miura, Y. et al. Mesenchymal stem cell-organized bone marrow elements: an alternative hematopoietic progenitor resource. Stem Cells 24, 2428–2436 (2006).
Meyer, L.H. & Debatin, K.M. Diversity of human leukemia xenograft mouse models: implications for disease biology. Cancer Res. 71, 7141–7144 (2011).
Manz, M.G. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26, 537–541 (2007).
Wunderlich, M. et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 24, 1785–1788 (2010).
Reinisch, A. et al. A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat. Med. 22, 812–821 (2016).
Reichert, J.C. et al. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials 31, 7928–7936 (2010).
Taubenberger, A.V., Quent, V.M., Thibaudeau, L., Clements, J.A. & Hutmacher, D.W. Delineating breast cancer cell interactions with engineered bone microenvironments. J. Bone Miner. Res. 28, 1399–1411 (2013).
Ding, L., Saunders, T.L., Enikolopov, G. & Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Raymaekers, K., Stegen, S., van Gastel, N. & Carmeliet, G. The vasculature: a vessel for bone metastasis. Bonekey Rep. 4, 742 (2015).
Yardeni, T., Eckhaus, M., Morris, H.D., Huizing, M. & Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab Anim. 40, 155–160 (2011).
Scepansky, E., Goldstein, R. & Rosenblatt, M. Preclinical orthotopic and intracardiac injection models of human breast cancer metastasis to bone and their use in drug discovery. Curr. Protoc. Pharmacol. 52, 14.18 (2011).
Park, S.I., Kim, S.J., McCauley, L.K. & Gallick, G.E. Pre-clinical mouse models of human prostate cancer and their utility in drug discovery. Curr. Protoc. Pharmacol. 51, 14.15 (2010).
Campbell, J.P., Merkel, A.R., Masood-Campbell, S.K., Elefteriou, F. & Sterling, J.A. Models of bone metastasis. J. Vis. Exp (67), e4260 (2012).
Barbier, V., Winkler, I.G. & Levesque, J.P. Mobilization of hematopoietic stem cells by depleting bone marrow macrophages. Methods Mol. Biol. 904, 117–138 (2012).
Acknowledgements
This work was supported by the Australian Research Council (Future Fellowship awarded to D.W.H.), the National Health and Medical Research Council (NHMRC Research Fellowship 1044091 to J.-P.L.; NHMRC Project Grant 1082313 to B.M.H., J.-P.L. and D.W.H.), the German Research Foundation (DFG HO 5068/1-1 to B.M.H., B.M.H. and DFG WA 3606/1-1 to F.W.), the National Breast Cancer Foundation (NBCF IN-15-047 to B.M.H. and D.W.H.) and a grant from Worldwide Cancer Research (WWCR 15-11563 to D.W.H.). We thank O. Ramuz, C. Theodoropoulos and J. Baldwin for their help and scientific input. Fibrin glue (TISSEEL Fibrin Sealant) was kindly provided by Baxter Healthcare International. The fibronectin (HFN 7.1) and osteonectin (AON-1) antibodies, developed by R.J. Klebe and J.D. Termine, respectively, were obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development of the National Institutes of Health, and were maintained at the University of Iowa Department of Biology.
Author information
Authors and Affiliations
Contributions
L.C.M., B.M.H., A.V.T., J.-P.L., P.Z., R.M. and D.W.H. conceived and designed the experiments. L.C.M., B.M.H., F.W., D.M., D.J.W., C.O.H., T.O., E.P., J.A.M. and B.N. performed the experiments and analyzed the data. L.C.M. wrote the manuscript with the assistance of B.M.H., C.V., E.M.D.-J.-P., F.M.W., J.-P.L., J.A.M. and R.M. C.V. established the CaP coating procedure. T.D.B., P.D.D. and D.W.H. designed and built the custom-made melt electrospinning machine. E.M.D.-J.-P., D.W.H. and F.M.W. developed the scaffold design and fabrication. V.M.Q., P.H. and A.V.T. conducted the first studies to establish the hTEBC model. D.W.H., A.V.T., B.M.H. and L.C.M. supervised the project. All authors read and critiqued the manuscript extensively.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Long term engraftment and multi-lineage reconstitution in mouse PB.
Flow cytometry gating strategy for hCD45+, hCD34+, CD19+, CD3+ and CD33+ cells. Cells were gated on living cells before cell doublets were excluded. Data was gated on all hCD45+ and mCD45+ cells were to account for hematopoietic lineages before sub-lineage populations were determined. Mouse peripheral blood (PB) and fluorescence minus one (FMO) controls from mice injected with 10,000 CD34+ cord blood cells (16 weeks) and implanted with hTEBC were used as examples of the flow cytometry gating strategy.
Supplementary Figure 2 Long term engraftment and multi-lineage reconstitution in mouse spleen.
Flow cytometry gating strategy for hCD45+, hCD34+, CD19+, CD3+ and CD33+ cells. Cells were gated on living cells before cell doublets were excluded. Data was gated on all hCD45+ and mCD45+ cells were to account for hematopoietic lineages before sub-lineage populations were determined. Cells isolated from mouse spleens and fluorescence minus one (FMO) controls from mice injected with 10,000 hCD34+ cord blood cells (16 weeks) and implanted with hTEBC were used as examples of the flow cytometry gating strategy.
Supplementary Figure 3 Long term engraftment and multi-lineage reconstitution in mouse femur.
Flow cytometry gating strategy for hCD45+, hCD34+, CD19+, CD3+ and CD33+ cells. Cells were gated on living cells before cell doublets were excluded. Data was gated on all hCD45+ and mCD45+ cells were to account for hematopoietic lineages before sub-lineage populations were determined. Analysis of bone marrow from mouse femurs (mBone) and fluorescence minus one (FMO) controls from mice injected with 10,000 hCD34+ cord blood cells (16 weeks) and implanted with hTEBC were used as examples of the flow cytometry gating strategy.
Supplementary Figure 4 Long term engraftment and multi-lineage reconstitution in hTEBC
Flow cytometry gating strategy for hCD45+, hCD34+, CD19+, CD3+ and CD33+ cells. Cells were gated on living cells before cell doublets were excluded. Data was gated on all hCD45+ and mCD45+ cells were to account for hematopoietic lineages before sub-lineage populations were determined. Analysis of bone marrow from the hTEBC and fluorescence minus one (FMO) controls from mice injected with 10,000 hCD34+ cord blood cells (16 weeks) and implanted with hTEBC were used as examples of the flow cytometry gating strategy. Note that the FMO controls are the same as used in Supplementary Figure 3.
Supplementary Figure 5 Multi-lineage reconstitution in mice peripheral blood and organs following hCD34+ cell engraftment
Mice were engrafted with either (a, c) 10,000 or (b, d) 50,000 hCD34+ cord blood cells. Flow cytometry analysis was performed and gated on the total population of mCD45+ and hCD45+ cells to detect (a, b) the time course of the relative hCD19+, hCD3+ and hCD33+ cell engraftment in mouse PB. (c, d) The organ distribution and engraftment of hCD19+, hCD3+ and hCD33+ cells within the total population of mCD45+ and hCD45+ cells at the experimental endpoint (16-24 weeks). Data is represented as mean ± s.e.m, n=3 for mice without a hTEBC and n=4 for mice with a hTEBC.
Supplementary Figure 6 Engraftment and multi-lineage reconstitution of mouse femur and hTEBC.
Flow cytometry gating strategy for hCD45+, hCD34+, hCD38+, hCD3+, hCD19+ and CD11b+ following engraftment with adult bone-derived hCD34+ cells. Analysis of bone marrow isolated from (a) mouse femur and (b) hTEBC from mice 5 weeks after injection with 2 × 105 hCD34+ cells.
Supplementary Figure 7 Controls for species-specificity of antibodies used for immunohistochemical analysis.
Human and mouse tissues were used to serve respectively as positive and negative controls to test the human-specificity of antibodies against human NuMA, lamin A/C, mitochondria, collagen type I, osteocalcin, CD146, CD45 and CD34. Details of antigen retrieval methods, antibody suppliers and antibody dilutions can be found in Supplementary Table 1.
Supplementary information
Supplementary Figures and Tables
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 1854 kb)
Rights and permissions
About this article
Cite this article
Martine, L., Holzapfel, B., McGovern, J. et al. Engineering a humanized bone organ model in mice to study bone metastases. Nat Protoc 12, 639–663 (2017). https://doi.org/10.1038/nprot.2017.002
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.002
This article is cited by
-
Osteoclasts differential-related prognostic biomarker for osteosarcoma based on single cell, bulk cell and gene expression datasets
BMC Cancer (2022)
-
Modelling skeletal pain harnessing tissue engineering
In vitro models (2022)
-
A humanized orthotopic tumor microenvironment alters the bone metastatic tropism of prostate cancer cells
Communications Biology (2021)
-
Materials design for bone-tissue engineering
Nature Reviews Materials (2020)
-
Engineering osteoblastic metastases to delineate the adaptive response of androgen-deprived prostate cancer in the bone metastatic microenvironment
Bone Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.