Abstract
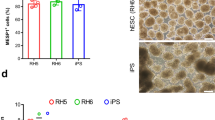
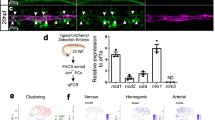
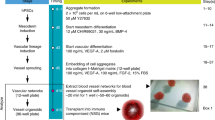
Human pluripotent stem cells (hPSCs) provide a valuable model for the study of human development and a means to generate a scalable source of cells for therapeutic applications. This protocol specifies cell fate efficiently into cardiac and endothelial lineages from hPSCs. The protocol takes 2 weeks to complete and requires experience in hPSC culture and differentiation techniques. Building on lessons taken from early development, this monolayer-directed differentiation protocol uses different concentrations of activin A and bone morphogenetic protein 4 (BMP4) to polarize cells into mesodermal subtypes that reflect mid-primitive-streak cardiogenic mesoderm and posterior-primitive-streak hemogenic mesoderm. This differentiation platform provides a basis for generating distinct cardiovascular progenitor populations that enable the derivation of cardiomyocytes and functionally distinct endothelial cell (EC) subtypes from cardiogenic versus hemogenic mesoderm with high efficiency without cell sorting. ECs derived from cardiogenic and hemogenic mesoderm can be matured into >90% CD31+/VE-cadherin+ definitive ECs. To test the functionality of ECs at different stages of differentiation, we provide methods for assaying the blood-forming potential and de novo lumen-forming activity of ECs. To our knowledge, this is the first protocol that provides a common platform for directed differentiation of cardiomyocytes and endothelial subtypes from hPSCs. This protocol yields endothelial differentiation efficiencies exceeding those of previously published protocols. Derivation of these cell types is a critical step toward understanding the basis of disease and generating cells with therapeutic potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Murry, C.E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Xu, P.F., Houssin, N., Ferri-Lagneau, K.F., Thisse, B. & Thisse, C. Construction of a vertebrate embryo from two opposing morphogen gradients. Science 344, 87–89 (2014).
Kattman, S.J. et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228–240 (2011).
Nostro, M.C., Cheng, X., Keller, G.M. & Gadue, P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell 2, 60–71 (2008).
Sumi, T., Tsuneyoshi, N., Nakatsuji, N. & Suemori, H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development 135, 2969–2979 (2008).
Palpant, N.J. et al. Inhibition of β-catenin signaling respecifies anterior-like endothelium into beating human cardiomyocytes. Development 142, 3198–209 (2015).
Palpant, N.J. et al. Transmembrane protein 88: a Wnt regulatory protein that specifies cardiomyocyte development. Development 140, 3799–3808 (2013).
Wamstad, J.A. et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 151, 206–220 (2012).
Willems, E. et al. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ. Res. 109, 360–364 (2011).
Mendjan, S. et al. NANOG and CDX2 pattern distinct subtypes of human mesoderm during exit from pluripotency. Cell Stem Cell 15, 310–325 (2014).
Faial, T. et al. Brachyury and SMAD signalling collaboratively orchestrate distinct mesoderm and endoderm gene regulatory networks in differentiating human embryonic stem cells. Development 142, 2121–2135 (2015).
Yutzey, K.E. & Bader, D. Diversification of cardiomyogenic cell lineages during early heart development. Circ. Res. 77, 216–219 (1995).
Van Handel, B. et al. Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 150, 590–605 (2012).
Rafii, S. et al. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood 121, 770–780 (2013).
Nourse, M.B. et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler. Thromb. Vasc. Biol. 30, 80–89 (2010).
Orlova, V.V. et al. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 9, 1514–1531 (2014).
Levenberg, S., Ferreira, L.S., Chen-Konak, L., Kraehenbuehl, T.P. & Langer, R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat. Protoc. 5, 1115–1126 (2010).
Kennedy, M., D'Souza, S.L., Lynch-Kattman, M., Schwantz, S. & Keller, G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109, 2679–2687 (2007).
White, M.P. et al. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells 31, 92–103 (2013).
Misfeldt, A.M. et al. Endocardial cells are a distinct endothelial lineage derived from Flk1+ multipotent cardiovascular progenitors. Dev. Biol. 333, 78–89 (2009).
Peterkin, T., Gibson, A. & Patient, R. Common genetic control of haemangioblast and cardiac development in zebrafish. Development 136, 1465–1474 (2009).
de la Pompa, J.L. et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392, 182–186 (1998).
Ranger, A.M. et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 392, 186–190 (1998).
Morikawa, Y. & Cserjesi, P. Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development 131, 2195–2204 (2004).
Barnes, R.M., Firulli, B.A., Conway, S.J., Vincentz, J.W. & Firulli, A.B. Analysis of the Hand1 cell lineage reveals novel contributions to cardiovascular, neural crest, extra-embryonic, and lateral mesoderm derivatives. Dev. Dyn. 239, 3086–3097 (2010).
Burridge, P.W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 109, E1848–E1857 (2012).
Kempf, H., Kropp, C., Olmer, R., Martin, U. & Zweigerdt, R. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat. Protoc. 10, 1345–1361 (2015).
Ditadi, A. et al. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat. Cell Biol. 17, 580–591 (2015).
Witty, A.D. et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat. Biotechnol. 32, 1026–1035 (2014).
Zhang, J. et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ. Res. 111, 1125–1136 (2012).
Paige, S.L. et al. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One 5, e11134 (2010).
Ueno, S. et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 9685–9690 (2007).
Mummery, C.L. et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 111, 344–358 (2012).
Lian, X., Zhang, J., Zhu, K., Kamp, T.J. & Palecek, S.P. Insulin inhibits cardiac mesoderm, not mesendoderm, formation during cardiac differentiation of human pluripotent stem cells and modulation of canonical Wnt signaling can rescue this inhibition. Stem Cells 31, 447–457 (2013).
Dubois, N.C. et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 29, 1011–1018 (2011).
Gantz, J.A. et al. Targeted genomic integration of a selectable floxed dual fluorescence reporter in human embryonic stem cells. PLoS One 7, e46971 (2012).
Ovchinnikov, D.A. et al. Isolation of contractile cardiomyocytes from human pluripotent stem-cell-derived cardiomyogenic cultures using a human NCX1-EGFP reporter. Stem Cells Dev. 24, 11–20 (2015).
Elliott, D.A. et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat. Methods 8, 1037–1040 (2011).
Tohyama, S. et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137 (2013).
Ciau-Uitz, A., Pinheiro, P., Gupta, R., Enver, T. & Patient, R. Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev. Cell 18, 569–578 (2010).
Ciau-Uitz, A., Pinheiro, P., Kirmizitas, A., Zuo, J. & Patient, R. VEGFA-dependent and -independent pathways synergise to drive Scl expression and initiate programming of the blood stem cell lineage in Xenopus. Development 140, 2632–2642 (2013).
Kennedy, M. et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2, 1722–1735 (2012).
Choi, K.D. et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2, 553–567 (2012).
Nakano, A., Nakano, H., Smith, K. & Palpant, N.J. The developmental origins and lineage contributions of endocardial endothelium. Biochim. Biophys. Acta 1863, 1937–1947 (2016).
Nakano, H. et al. Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat. Commun. 4, 1564 (2013).
Sturgeon, C.M., Ditadi, A., Awong, G., Kennedy, M. & Keller, G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 32, 554–561 (2014).
Zheng, Y. et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 109, 9342–9347 (2012).
Acknowledgements
This work was supported by the following grants: PO1 GM081619, PO1 HL094374, RO1 HL084642, and UO1 HL100405 (C.E.M.); NIH DP2DK102258-01 (Y.Z.); T32 EB001650 (R.J.Z.); NIH T32EB001650 and NIH T32HL007312 (M.R.); NIH UO1 HL100395 and U01 HL099997 (I.B.); and Alex's Lemonade Stand Young Investigator Award and the Hyundai Hope Scholar Grant (B.H.).
Author information
Authors and Affiliations
Contributions
N.J.P. conceptually developed protocols, designed and performed experiments, analyzed data, and wrote the manuscript. L.P. conceptually developed protocols, designed experiments, and assisted in preparation of the manuscript. C.E.F., M.R., B.H., and R.J.Z. performed experiments, analyzed data, and helped prepare protocol details. Y.Z. and I.B. designed experiments and assisted in preparation of the manuscript. C.E.M. supervised the project, obtained funding for the research, and wrote and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Protocol development for directed differentiation of endothelium from human embryonic stem cells.
(a) Schematic diagram of time course of directed differentiation and days (a-f) in which changes were made as outlined in panels b-j to optimize conditions for differentiation into endothelium. (b-j) Flow cytometry analysis for the cardiac progenitor marker KDR+/PDGFRα+ and the endothelial marker KDR+/CD31+ (b-h) at day 5 of differentiation. Manipulation of conditions at time points a-f are described to the right of each panel.
Supplementary Figure 2 Time dependency of VEGF treatment during endothelial differentiation.
(a) Schematic of differentiation timeline. (b) Exposure to VEGF continuously starting on day 1 of differentiation vs. subsequent days as it impacts efficiency of endothelial cell production on day 5 by FACS analysis for KDR and CD34. (c) Exposure to VEGF for 24 hour increments starting on day 1 vs. subsequent days as it impacts efficiency of endothelial cell production on day 5 by FACS analysis for KDR and CD34.
Supplementary Figure 3 Examples of FACS profiles for analysis
(a-d) FSC/SSC plot (left) isotype gating (middle) and experimental gating (right) for all analyses reported in the protocol including cTnT (a), CD31 (b), CD43/CD235a (c) and KDR/CD34 (d).
Supplementary information
Supplementary Figures and Text
Supplementary Figures 1–3 and Supplementary Methods (PDF 929 kb)
Rights and permissions
About this article
Cite this article
Palpant, N., Pabon, L., Friedman, C. et al. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat Protoc 12, 15–31 (2017). https://doi.org/10.1038/nprot.2016.153
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.153
This article is cited by
-
Targeted CRISPR activation is functional in engineered human pluripotent stem cells but undergoes silencing after differentiation into cardiomyocytes and endothelium
Cellular and Molecular Life Sciences (2024)
-
Cellular heterogeneity of pluripotent stem cell-derived cardiomyocyte grafts is mechanistically linked to treatable arrhythmias
Nature Cardiovascular Research (2024)
-
The Role of Large Animal Models in Cardiac Regeneration Research Using Human Pluripotent Stem Cell-Derived Cardiomyocytes
Current Cardiology Reports (2023)
-
Application of Human Induced Pluripotent Stem Cells for Tissue Engineered Cardiomyocyte Modelling
Regenerative Engineering and Translational Medicine (2023)
-
PAX3-FOXO1 dictates myogenic reprogramming and rhabdomyosarcoma identity in endothelial progenitors
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.