Abstract
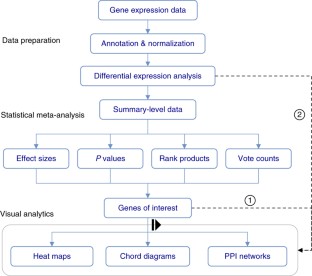
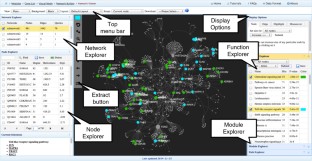
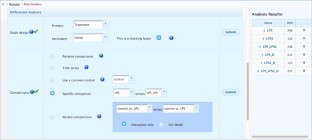
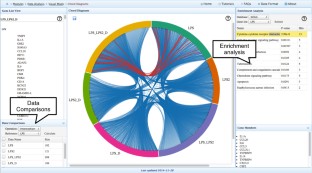
Meta-analysis of gene expression data sets is increasingly performed to help identify robust molecular signatures and to gain insights into underlying biological processes. The complicated nature of such analyses requires both advanced statistics and innovative visualization strategies to support efficient data comparison, interpretation and hypothesis generation. NetworkAnalyst (http://www.networkanalyst.ca) is a comprehensive web-based tool designed to allow bench researchers to perform various common and complex meta-analyses of gene expression data via an intuitive web interface. By coupling well-established statistical procedures with state-of-the-art data visualization techniques, NetworkAnalyst allows researchers to easily navigate large complex gene expression data sets to determine important features, patterns, functions and connections, thus leading to the generation of new biological hypotheses. This protocol provides a step-wise description of how to effectively use NetworkAnalyst to perform network analysis and visualization from gene lists; to perform meta-analysis on gene expression data while taking into account multiple metadata parameters; and, finally, to perform a meta-analysis of multiple gene expression data sets. NetworkAnalyst is designed to be accessible to biologists rather than to specialist bioinformaticians. The complete protocol can be executed in ∼1.5 h. Compared with other similar web-based tools, NetworkAnalyst offers a unique visual analytics experience that enables data analysis within the context of protein-protein interaction networks, heatmaps or chord diagrams. All of these analysis methods provide the user with supporting statistical and functional evidence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Li, S. et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 15, 195–204 (2014).
Pena, O.M. et al. An endotoxin tolerance signature predicts sepsis and organ dysfunction at initial clinical presentation. EBioMedicine 1, 64–71 (2014).
Zhang, G. et al. Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effects in human pancreatic cancer. Clin. Cancer Res. 19, 4983–4993 (2013).
Gieger, C. et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet. 4, e1000282 (2008).
Gomez-Cabrero, D. et al. Data integration in the era of omics: current and future challenges. BMC Syst. Biol. 8 (suppl. 2), I1 (2014).
Tseng, G.C., Ghosh, D. & Feingold, E. Comprehensive literature review and statistical considerations for microarray meta-analysis. Nucleic Acids Res. 40, 3785–3799 (2012).
O'Donoghue, S.I. et al. Visualizing biological data-now and in the future. Nat. Methods 7, S2–S4 (2010).
Goble, C. & Stevens, R. State of the nation in data integration for bioinformatics. J. Biomed. Inform. 41, 687–693 (2008).
Smith, B. et al. The OBO Foundry: coordinated evolution of ontologies to support biomedical data integration. Nat. Biotechnol. 25, 1251–1255 (2007).
Wodke, J.A. et al. MyMpn: a database for the systems biology model organism Mycoplasma pneumoniae. Nucleic Acids Res. 43 (Database issue): D618–D623 (2014).
Breuer, K. et al. InnateDB: systems biology of innate immunity and beyond—recent updates and continuing curation. Nucleic Acids Res. 41, D1228–D1233 (2013).
Rhodes, D.R. et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9, 166–180 (2007).
Chelaru, F., Smith, L., Goldstein, N. & Bravo, H.C Epiviz: interactive visual analytics for functional genomics data. Nat. Methods 11, 938–940 (2014).
Nekrutenko, A. & Taylor, J. Next-generation sequencing data interpretation: enhancing reproducibility and accessibility. Nat. Rev. Genet. 13, 667–672 (2012).
Xia, J. et al. INMEX—a web-based tool for integrative meta-analysis of expression data. Nucleic Acids Res. 41, W63–W70 (2013).
Xia, J., Lyle, N.H., Mayer, M.L., Pena, O.M. & Hancock, R.E.W. INVEX—a web-based tool for integrative visualization of expression data. Bioinformatics 29, 3232–3234 (2013).
Xia, J., Benner, M.J. & Hancock, R.E.W. NetworkAnalyst—integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 42, W167–W174 (2014).
Tarraga, J. et al. GEPAS, a web-based tool for microarray data analysis and interpretation. Nucleic Acids Res. 36, W308–W314 (2008).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Xia, J., Mandal, R., Sinelnikov, I.V., Broadhurst, D. & Wishart, D.S. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 40, W127–W133 (2012).
Xia, J. & Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 6, 743–760 (2011).
Saeed, A.I. et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34, 374–378 (2003).
Perez-Llamas, C. & Lopez-Bigas, N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS ONE 6, e19541 (2011).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Huang, D.W. et al. DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 35, W169–W175 (2007).
Reimand, J., Arak, T. & Vilo, J. g:Profiler—a web server for functional interpretation of gene lists (2011 update). Nucleic Acids Res. 39, W307–W315 (2011).
Lynn, D.J. et al. InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Mol. Syst. Biol. 4, 218 (2008).
Saito, R. et al. A travel guide to Cytoscape plugins. Nat. Methods 9, 1069–1076 (2012).
Smyth, G.K. Limma: linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor (eds. Gentleman, R. et al. 397–420 (Springer, 2005).
Law, C.W., Chen, Y., Shi, W. & Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Orchard, S. et al. Protein interaction data curation: the International Molecular Exchange (IMEx) consortium. Nat. Methods 9, 345–350 (2012).
Turinsky, A.L., Razick, S., Turner, B., Donaldson, I.M. & Wodak, S.J. Interaction databases on the same page. Nat. Biotechnol. 29, 391–393 (2011).
Bader, G.D., Betel, D. & Hogue, C.W. BIND: the Biomolecular Interaction Network Database. Nucleic Acids Res. 31, 248–250 (2003).
Stark, C. et al. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. 34, D535–D539 (2006).
Licata, L. et al. MINT, the molecular interaction database: 2012 update. Nucleic Acids Res. 40, D857–D861 (2012).
Hermjakob, H. et al. IntAct: an open source molecular interaction database. Nucleic Acids Res. 32, D452–D455 (2004).
Rolland, T. et al. A proteome-scale map of the human interactome network. Cell 159, 1212–1226 (2014).
Pena, O.M., Pistolic, J., Raj, D., Fjell, C.D. & Hancock, R.E.W. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J. Immunol. 186, 7243–7254 (2011).
Ramasamy, A., Mondry, A., Holmes, C.C. & Altman, D.G. Key issues in conducting a meta-analysis of gene expression microarray datasets. PLoS Med. 5, e184 (2008).
Mitra, K., Carvunis, A.R., Ramesh, S.K. & Ideker, T. Integrative approaches for finding modular structure in biological networks. Nat. Rev. Genet. 14, 719–732 (2013).
Ideker, T., Ozier, O., Schwikowski, B. & Siegel, A.F. Discovering regulatory and signalling circuits in molecular interaction networks. Bioinformatics 18 (suppl. 1), S233–S240 (2002).
Beisser, D., Klau, G.W., Dandekar, T., Muller, T. & Dittrich, M.T. BioNet: an R package for the functional analysis of biological networks. Bioinformatics 26, 1129–1130 (2010).
Vidal, M., Cusick, M.E. & Barabasi, A.L. Interactome networks and human disease. Cell 144, 986–998 (2011).
Yu, H. et al. High-quality binary protein interaction map of the yeast interactome network. Science 322, 104–110 (2008).
Schramm, S.J. et al. Disturbed protein-protein interaction networks in metastatic melanoma are associated with worse prognosis and increased functional mutation burden. Pigment Cell Melanoma Res. 26, 708–722 (2013).
Liu, Y., Koyuturk, M., Barnholtz-Sloan, J.S. & Chance, M.R. Gene interaction enrichment and network analysis to identify dysregulated pathways and their interactions in complex diseases. BMC Syst. Biol. 6, 65 (2012).
Krzywinski, M. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009).
Bastian, M., Heymann, S. & Jacomy, M. Gephi: an open source software for exploring and manipulating networks. in International AAAI Conference on Weblogs and Social Media http://www.aaai.org/ocs/index.php/ICWSM/09/paper/viewFile/154Forum/1009 (2009).
Acknowledgements
The authors thank the Canadian Institutes for Health Research (CIHR) for financial support.
Author information
Authors and Affiliations
Contributions
J.X. developed NetworkAnalyst and prepared the protocol, E.E.G. tested the tool and the protocol and R.E.W.H. participated in all processes. All authors have read and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xia, J., Gill, E. & Hancock, R. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc 10, 823–844 (2015). https://doi.org/10.1038/nprot.2015.052
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.052
This article is cited by
-
Single-cell and transcriptomic analyses reveal the influence of diabetes on ovarian cancer
BMC Genomics (2024)
-
Identification of shared pathogenetic mechanisms between COVID-19 and IC through bioinformatics and system biology
Scientific Reports (2024)
-
A Multistep In Silico Approach Identifies Potential Glioblastoma Drug Candidates via Inclusive Molecular Targeting of Glioblastoma Stem Cells
Molecular Neurobiology (2024)
-
ERBB2-PTGS2 axis promotes intervertebral disc degeneration by regulating senescence of nucleus pulposus cells
BMC Musculoskeletal Disorders (2023)
-
An updated C. elegans nuclear body muscle transcriptome for studies in muscle formation and function
Skeletal Muscle (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.