Abstract
This review provides an overview of the changing US epidemiology of cannabis use and associated problems. Adults and adolescents increasingly view cannabis as harmless, and some can use cannabis without harm. However, potential problems include harms from prenatal exposure and unintentional childhood exposure; decline in educational or occupational functioning after early adolescent use, and in adulthood, impaired driving and vehicle crashes; cannabis use disorders (CUD), cannabis withdrawal, and psychiatric comorbidity. Evidence suggests national increases in cannabis potency, prenatal and unintentional childhood exposure; and in adults, increased use, CUD, cannabis-related emergency room visits, and fatal vehicle crashes. Twenty-nine states have medical marijuana laws (MMLs) and of these, 8 have recreational marijuana laws (RMLs). Many studies indicate that MMLs or their specific provisions did not increase adolescent cannabis use. However, the more limited literature suggests that MMLs have led to increased cannabis potency, unintentional childhood exposures, adult cannabis use, and adult CUD. Ecological-level studies suggest that MMLs have led to substitution of cannabis for opioids, and also possibly for psychiatric medications. Much remains to be determined about cannabis trends and the role of MMLs and RMLs in these trends. The public, health professionals, and policy makers would benefit from education about the risks of cannabis use, the increases in such risks, and the role of marijuana laws in these increases.
Similar content being viewed by others
Main
Cannabis has been used in the United States since the 1800s, with public attitudes toward its acceptability and potential harmfulness varying over time (Musto, 1991). Since 1996, US state laws about the legal use of cannabis for medical and recreational purposes have changed, as have public attitudes about the safety and acceptability of cannabis use. This review aims to provide a broad overview of the epidemiology of cannabis use and associated problems in the United States. The review begins with a section on epidemiologic and public health findings about adverse behavioral, psychosocial, and psychiatric problems associated with cannabis use. These are presented in life-course order of cannabis exposure: prenatal period, childhood, adolescence, and adulthood. The section includes consideration of whether the associations are causal or not (Hall et al, 2016), eg, ruling out reverse causation by clarifying time order (ensuring that the problem does not precede cannabis use) and confounding (controlling for factors that increase the likelihood of both using cannabis and developing the problem). The second section covers changes in the public perception of potential harms/adverse consequences. In the third section, time trends in cannabis use and behavioral, psychosocial, and psychiatric problems are reviewed. The fourth section covers available evidence about the influence of state medical and recreational marijuana laws on the prevalence of cannabis use and associated problems, as well as on substitution of cannabis for opioids, alcohol, and psychiatric medications. Finally, implications for clinicians, policy makers, and the public are considered, and future research directions suggested.
Adverse health and psychosocial problems associated with cannabis use
Prenatal Exposure
Many concerns exist about maternal use of cannabis during pregnancy and potential harm to the fetus (Volkow et al, 2017). Consistent with this, the American College of Obstetricians and Gynecologists recommends advising pregnant women and women contemplating pregnancy about potential risks of prenatal marijuana use in order to discourage use (American College of Obstetricians and Gynecologists Committee on Obstetric Practice, 2015). In 2007–2012 data from the National Surveys on Drug Use and Health (Ko et al, 2015), 3.9% of pregnant women used cannabis in the past month, 7.0% used it in the past 2–12 months, and among past-year users, 16.2% used near daily (Ko et al, 2015). A recent meta-analysis indicates that infants born to women who used marijuana prenatally were more likely than others to be anemic, have low birth weight, and require neonatal intensive care (Gunn et al, 2016). Prenatal marijuana exposure is also linked to subsequent impaired executive functioning in school (Wu et al, 2011), consistent with prospective research showing associations between prenatal cannabis exposure, restricted fetal growth, and greater childhood frontal cortical thickness (El Marroun et al, 2016). However, understanding whether prenatal cannabis exposure is causally related to poor childhood outcomes is complicated by the fact that most pregnant cannabis users in existing studies also used other substances, limiting knowledge about effects specific to cannabis (National Academies of Sciences, 2017; Volkow et al, 2017). Media reports suggest that, increasingly, some women see cannabis as a natural, safe substance to use throughout pregnancy, even if they do not use other substances (The New York Times, 2017). Such trends are concerning given that risks may well exist, although the need for further research on cannabis use and pregnancy outcomes is clear.
Childhood Exposure
The onset of recreational cannabis use almost always begins in adolescence, with ‘early-onset’ generally referring to early adolescence, not childhood. Consistent with this, the national Monitoring The Future (MTF) study found that in 2016, the prevalence of cannabis use among eighth graders (generally age 13–14 years) was only 5.4% (The Monitoring the Future study and the University of Michigan, 2016). Therefore, the main type of cannabis exposure in childhood is unintentional, often resulting from ingestion of cannabis or a cannabis product. Although the literature does not show evidence of fatal cannabis exposures (in children or adults), acute symptoms in children can include lethargy, ataxia, dizziness, and respiratory depression (National Academies of Sciences, 2017). A recent review indicated increasing risk for pediatric cannabis exposures, especially in states with legal cannabis use (National Academies of Sciences, 2017) (see below).
Adolescent Exposure
Concerns about the risks of adolescent cannabis use, especially regular or heavy use, focus on the developing adolescent brain (Batalla et al, 2013; Volkow et al, 2014; Zalesky et al, 2012), poor educational outcome (Fergusson et al, 2015), school dropout, cognitive impairment and lower IQ (Meier et al, 2012), lower life satisfaction and achievement (Fergusson and Boden, 2008), and addiction (Agrawal et al, 2004; Chen et al, 2009). However, studies of these problems, particularly those related to cognitive functioning, are not considered conclusive (National Academies of Sciences, 2017) because shared risk factors could be responsible for both the early cannabis use and the impairments shown later (Volkow et al, 2014). Furthermore, cognitive impairment could predate the earliest cannabis use. For this reason, the large-scale Adolescent Brain Cognitive Development (ABCD) Study (National Institutes of Health, 2015) has been launched. The ABCD study aims to conduct extensive neurocognitive and brain imaging studies on children before the earliest age at onset of cannabis use, and then to repeatedly assess children who do and do not use cannabis over 10 years to determine their neurocognitive and other outcomes.
Adult Exposure
Overall mortality and fatal overdose
A number of studies have been conducted on the relationship between cannabis use and overall mortality. Unadjusted analyses show an association between cannabis use at a given point in time and overall mortality years later (National Academies of Sciences, 2017). However, after adjustment, associations are generally reduced or eliminated. Many problems exist with these data and study designs, making determination of a causal relationship difficult (National Academies of Sciences, 2017). A further complication to this research is that the cause of death noted in the mortality statistics does not always reflect acute cannabis use, as suggested by toxicology results indicating cannabis use in a series of hanging deaths (San Nicolas and Lemos, 2015) in San Francisco. There are no known cases of fatal overdose from cannabis use in the epidemiologic literature (Calabria et al, 2010; Hall et al, 2016).
An important type of harm related to cannabis use is the increased risk for injury or fatality due to intoxication while driving (Hall et al, 2016). The primary psychoactive component of cannabis, Δ-9-tetrahydrocannabinol (THC), impairs the motor and cognitive functions needed for safe driving (Ramaekers et al, 2004; Rogeberg and Elvik, 2016), making clear the causal role of cannabis in this public health problem. Cannabis use while driving has been shown to substantially increase the risk for motor vehicle crashes (Asbridge et al, 2012; Brady and Li, 2014; Li et al, 2012; National Academies of Sciences, 2017; Rogeberg and Elvik, 2016), and is implicated in fatal and nonfatal crashes (Asbridge et al, 2012; Brady and Li, 2014; Hartman and Huestis, 2013; Li et al, 2012; Ramaekers et al, 2004; Zhu and Wu, 2016). Injury and fatality risk for crashes may be further increased because of a link between cannabis use and failure to use seatbelts (Liu et al, 2016). In Canada, where medical marijuana has been legal since 2001, cannabis-attributable driving harms and costs are substantial (Wettlaufer et al, 2017). In addition to motor vehicle crashes, cannabis has also been implicated in fatal injuries among US pilots (McKay and Groff, 2016).
Legislation that prohibits driving while under the influence of alcohol is enforceable because roadside breathalyzer tests can detect whether a driver has exceeded a legal blood alcohol concentration (BAC) limit indicating impairment that is standardized nationwide. In all 50 states, this limit is a BAC level of 0.08%, whereas for commercial drivers, a lower BAC of 0.04% is used. Furthermore, ignition interlocks, or alcohol-sensing devices connected to a vehicle’s ignition to prevent it from starting if a driver has or exceeds a predetermined BAC level, are a promising avenue for preventing alcohol-involved driving risks, as they reduce fatal vehicle crashes among repeat DUI offenders (McGinty et al, 2017b). Unfortunately, no parallel tests or devices exist for cannabis. Cannabis metabolites can be detected in blood, blood plasma, oral fluid, and urine (Lee et al, 2013; Marsot et al, 2016), although the presence of such metabolites does not necessarily indicate the likelihood of acute intoxication, as BAC does. An accurate ‘breathalyzer’ test for cannabis that could be used on a widespread basis has not yet been developed. This area is greatly in need of scientific advancement. Nevertheless, roadside drug testing using various methods of testing has been introduced in a number of countries (Watson and Mann, 2016), and several US states that have legalized cannabis use, eg, California, Colorado, Oregon, and Massachusetts, are experimenting with different forms of roadside testing for driving under the influence of cannabis, including biological and behavioral tests (eg, asking drivers to indicate their ability to balance).
Addiction/substance use disorder
The Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association includes definitions of substance use disorders (SUD), including cannabis use disorder (CUD). The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; American Psychiatric Association, 1994) was published in 1994, and was in use until 2013, when Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; American Psychiatric Association, 2013) was published. Thus, although DSM-5 is more recent, DSM-IV definitions formed the basis of a large body of research.
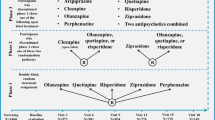
For substance use disorders, including cannabis use disorders, DSM-IV defined two disorders, dependence and abuse (Figure 1). DSM-IV definition of dependence was similar to dependence diagnosis in the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10; World Health Organization, 1992), and showed high empirical agreement in the US and international studies (Grant, 1996; Hasin et al, 1997, 2006; Pull et al, 1997; Rounsaville et al, 1993; Ustun et al, 1997). ICD-10 had no corresponding category for DSM-IV ‘abuse’, but instead, a ‘hazardous use’ category. Abuse and hazardous use had poor empirical agreement (Grant, 1996; Hasin et al, 1997, 2006; Pull et al, 1997; Rounsaville et al, 1993; Ustun et al, 1997). Many studies in adolescents and adults examined the DSM-IV distinction between dependence and abuse. Results were very consistent: abuse and dependence criteria formed a single, unidimensional construct (Hasin et al, 2013).
DSM-IV and DSM-5 criteria for cannabis use disorder (CUD).
In DSM-5, most criteria for abuse and dependence were combined into a single disorder (Figure 1). Additional DSM-5 changes included removing the DSM-IV legal problems criterion, and adding criteria for craving and cannabis withdrawal (Hasin et al, 2013). In contrast to DSM-5, ICD-11 will retain dependence as the ‘master diagnosis’ (Saunders, 2017). The differences between DSM-5 and ICD-11 will doubtless generate discussion and additional studies (Saunders, 2017).
A common assumption about the risk for CUD among users is that it is rare, based on findings from 25 years ago that few cannabis users developed CUD (Anthony et al, 1994; Joy et al, 2017). However, in more recent US national data, 3 out of 10 cannabis users developed DSM-IV CUD (Hasin et al, 2015a). Moreover, extending analyses of DSM-5 diagnoses of CUD (Hasin et al, 2016), 19.5% of lifetime users met criteria for DSM-5 CUD, of whom 23% were symptomatically severe (⩾6 criteria). Of these, 48% were not functioning in any major role (eg, work). Thus, CUD in users is not rare and can be serious.
In terms of causality, cannabis use is clearly a necessary condition for CUD, but as not all cannabis users develop CUD, use is not clearly sufficient. The etiology of CUD is complex (Agrawal and Lynskey, 2006; Bogdan et al, 2016; Haberstick et al, 2011; Verweij et al, 2013b), involving both genetic (Sherva et al, 2016) and environmental factors. Social-ecological models of substance use (Babor, 2010; Connell et al, 2010; Corbett, 2001; Gruenewald, 2011; Gruenewald et al, 2014) assume that in general, use is increased by factors that increase availability and also desirability, by normalizing use and reducing perception of harm. If these environmental factors also increase the prevalence of heavy or frequent users, then they are likely to increase the risk for CUD.
Cannabis withdrawal
When DSM-IV was published, little was known about cannabis withdrawal. Since then, preclinical (Copersino et al, 2006; Goldstein and Volkow, 2011; Haney et al, 2004; Martinez et al, 2007), clinical (Agrawal et al, 2008; Budney and Hughes, 2006; Budney et al, 2004; Chung et al, 2008; Copersino et al, 2006; Goldstein and Volkow, 2011; Hasin et al, 2008; Martinez et al, 2007), and epidemiologic (Agrawal et al, 2008; Hasin et al, 2008) studies have demonstrated a cannabis withdrawal syndrome after cessation of use. This syndrome is most intense during the first week of abstinence, but can persist as long as a month after use (Budney et al, 2003; Copersino et al, 2006; Elkashef et al, 2008; Hall et al, 2016; Kouri and Pope, 2000; Milin et al, 2008) and has pharmacological specificity (Budney et al, 2007; Haney et al, 2004; Lichtman and Martin, 2002). Cannabis withdrawal is reported by up to one-third of regular users in the general population (Agrawal et al, 2008; Budney and Hughes, 2006; Hasin et al, 2008) and by 50–95% among heavy users in treatment or research studies (Chung et al, 2008; Copersino et al, 2006; Cornelius et al, 2008; Levin et al, 2010). The clinical significance of cannabis withdrawal is shown by the fact that it can be impairing (Allsop et al, 2012), that cannabis or other substances are used to relieve it, and by its association with trouble quitting use (Budney et al, 2008; Copersino et al, 2006; Levin et al, 2010) and worse treatment outcomes (Allsop et al, 2012; Chung et al, 2008; Cornelius et al, 2008; Greene and Kelly, 2014). In addition, in latent variable modeling (Agrawal and Lynskey, 2007), adding withdrawal to other CUD criteria improves model fit. In terms of etiology, cannabis withdrawal is moderately heritable (Verweij et al, 2013a), implicating both genetic and environmental factors.
Cannabis withdrawal is defined as three or more of the symptoms shown in Table 1 following cessation of prolonged use (American Psychiatric Association, 2013). Many of these symptoms overlap with symptoms of depressive or anxiety disorders. Withdrawal from other substances (eg, alcohol, opioids) is widely recognized (Stern et al, 2010). However, many professionals and members of the general public may not be aware of cannabis withdrawal (Katz et al, 2014), potentially leading to confusion about the benefits of cannabis to treat or self-medicate symptoms of anxiety or depressive disorders (see below).
Psychiatric comorbidity: mood, anxiety, and other substance use disorders
In 2001–2002 data from the US National Epidemiologic Survey on Alcohol and Related Conditions (NESARC; for more information, see Table 2), strong associations were found between DSM-IV cannabis use disorders and other substance and psychiatric disorders (Stinson et al, 2006), including alcohol and nicotine use disorders, mood disorders, anxiety disorders, personality disorders, and posttraumatic stress disorder (PTSD). Associations were also found between DSM-IV CUD and alcohol and nicotine dependence in data from the National Survey on Drug Use and Health (Wu et al, 2017) (NSDUH; Table 2), a series of national surveys of US household residents aged ⩾12 years. In a national database of hospitalized patients, ICD-9-CM CUD diagnoses were associated with schizoaffective/mood disorders and alcohol use disorders (Charilaou et al, 2017). In military veterans with ICD-9-CM CUD treated in the Veterans Health Administration, PTSD was the most common psychiatric comorbidity (Bujarski et al, 2016). In the 2012–2013 NESARC survey (NESARC-III, for more information, see Table 2), the comorbidity of CUD and psychiatric disorders was reexamined using DSM-5 definitions (Hasin et al, 2016). Again, strong, significant associations were found with other substance use disorders (alcohol, drug, nicotine), mood, anxiety, personality disorders, and PTSD (Hasin et al, 2016). Using DSM-5 CUD severity definitions of mild, moderate, and severe, associations with psychiatric disorders were stronger with more severe levels of CUD (Hasin et al, 2016).
Whether the relationship between cannabis use or CUD and mood or anxiety disorders is causal or not has been debated (Agrawal and Lynskey, 2014). The reasons for the strong, significant associations of CUD with mood and anxiety disorders could be because of shared common etiology, cannabis use or CUD leading to mood or anxiety disorders, or mood or anxiety disorders leading to cannabis use and subsequently to CUD. A prospective study using NESARC data suggested that after controlling for a multitude of potential cofactors, cannabis use predicted incidence of other substance use disorders but not mood or anxiety disorders (Blanco et al, 2016). A study of polygenic risk suggested that cannabis use or CUD shared genetic risk with major depression (Carey et al, 2016), as did a study of cannabis dependence using a genome-wide association approach (Sherva et al, 2016). Additional genetic studies suggest either common causes underlying the comorbidity between CUD and major depression (Hodgson et al, 2017) or a causal effect of CUD on major depression (Smolkina et al, 2017). Thus, the nature of the relationship between cannabis use or CUD and psychiatric comorbidity remains a topic of debate.
Psychiatric comorbidity: psychotic disorders
Cannabis use and psychosis are associated (Charilaou et al, 2017). For example, in 30 studies of healthy controls and ultra-high-risk (UHR) individuals (with subclinical psychotic symptoms and/or genetic risk and impaired functioning), a meta-analysis (Carney et al, 2017) showed that UHR individuals had higher rates of cannabis use and CUD, and UHR cannabis users had higher rates than nonusers of positive psychotic symptoms (unusual thought content, suspiciousness). Epidemiologic efforts to determine causation have focused on long-term prospective studies in which the time order of cannabis use and onset of psychosis indicators can be determined. For example, in 1265 children born in Christchurch, New Zealand, in 1977 assessed repeatedly, daily cannabis users later had higher rates of psychotic symptoms at age 18–25 years, controlling for many fixed and dynamic confounders (Boden et al, 2007). In this sample, structural equation modeling showed a significant pathway from cannabis to psychosis, but not from psychosis to cannabis use (Fergusson et al, 2015), supporting a causal relationship of cannabis use to development of psychotic symptoms. In a recent review (Gage et al, 2016), 10 prospective studies were considered, including a national record study of >50 000 male Swedish conscripts (Zammit et al, 2002). The review found a significant relationship between earlier cannabis use and later development of psychosis, and called for studies to determine the effect of different strains of cannabis on risk, and to identify particularly susceptible high-risk groups (Gage et al, 2016). A meta-analysis of 7 prospective studies of UHR samples (Kraan et al, 2016) did not support a relationship between cannabis use and psychosis onset. However, 5 of the studies ascertained CUD diagnoses at baseline, and in these studies, CUD was a significant predictor of subsequent psychosis. Both reviews of prospective studies (Gage et al, 2016; Kraan et al, 2016) noted concerns about elevated risk from high-THC cannabis. Although some debate remains about the causal link between cannabis and psychosis (Haney and Evins, 2016), a comprehensive review of reviews concluded that cannabis use is likely to increase psychosis risk, with increasing levels of use leading to increased risk (National Academies of Sciences, 2017). Furthermore, cannabis use has been characterized as one of the strongest modifiable risk factors for developing a psychotic disorder, with a recommendation that a child or teen with a family history of psychosis or prodromal symptoms should be informed of the risks and counseled strongly not to use cannabis (Weiss et al, 2017). As the legalized recreational market in the US increasingly distributes stronger forms of cannabis, eg, vaping, dabbing, and rosin (Baumann and Scheinbaum, 2016), further studies of the relationship of cannabis to psychosis are warranted, as well as public and professional education about the risks.
Psychiatric comorbidity: nicotine use disorders
The association of cannabis and nicotine use disorders merits attention because of the most common route of administration of both substances, which is smoking, and also because cannabis and nicotine co-use can intensify cannabis effects (Penetar et al, 2005; Rabin and George, 2015; Wang et al, 2016b), although not all studies agree on this point (Haney et al, 2013). Individuals who use both cannabis and tobacco have greater risk of respiratory distress than cannabis-only users or tobacco-only users (Agrawal et al, 2012), although cannabis has not been shown to be associated with increased rates of lung cancer (Tashkin, 2015). The co-occurrence of cannabis and tobacco use could have many possible explanations including predisposing genetic factors, peer influences, availability, and social milieu (Agrawal et al, 2012).
Trends in public perceptions
Inverse Relationship between Perceived Harmfulness of Cannabis and Likelihood of Use
Perceived harmfulness of cannabis has long been considered an important factor preventing adolescent cannabis use (Janz and Becker, 1984; Keyes et al, 2016; Piontek et al, 2013; Schmidt et al, 2016). MTF, a series of annual national school surveys of US adolescents in eighth, tenth, and twelfth grades (13–14, 15–16, and 17–18 years of age; see Table 2 for more information about the MTF surveys) provided the earliest evidence of an inverse relationship between perceived harmfulness and cannabis use (Bachman et al, 1988, 1998; Johnston et al, 1981). This relationship has now been shown repeatedly (Pacek et al, 2015; Swaim, 2003), including an international study in 32 countries (Piontek et al, 2013). An inverse relationship between perceived harmfulness and cannabis use has also been demonstrated for US adults in the annual NSDUH (see Table 2 for more information about the NSDUH surveys; (Compton et al, 2016).
Increasing Public Perception That Cannabis Is Harmless
Among US students in the MTF surveys of students, the perception that regular use of cannabis is risky declined substantially since 2004–2005 (The Monitoring the Future study and the University of Michigan, 2016); rates of decline ranged from 50 to 80%. In 2016, only ∼30% of twelfth graders perceived such risk. The perception that cannabis use is risky also declined among adults. Among NSDUH adult participants, the perception that cannabis use involves great risk declined from 50 to 33% between 2002 and 2014, whereas the perception that use involves no risk increased from ∼6 to 15% (Compton et al, 2016).
Trends in cannabis potency, use, and associated problems
Time Trends in Cannabis Potency
Over the past four decades, cannabis potency, indicated by the THC content in seized samples, has approximately doubled worldwide (Cascini et al, 2012; Hall et al, 2016), including in the United States (ElSohly et al, 2016). In the early 1990s, the average THC content in confiscated US marijuana samples was ~3.7%, whereas in 2014 it was ~6.1% (National Institute on Drug Abuse, 2017). This increase is relevant to epidemiologic study of time trends in problems associated with cannabis use because cannabis with higher THC concentrations is more likely to increase risks associated with use (Englund et al, 2017). However, in states that pass recreational marijuana laws, the potency of legally purchased cannabis may be more relevant to public health (see below).
Time Trends in Prenatal Cannabis Exposure
Among NSDUH participants aged 18–44 years who were pregnant, the estimated prevalence of past-month cannabis use increased 62% between 2002 and 2014, from 2.4 to 3.9% (Brown et al, 2017). In 2014, the rate of past-month cannabis use was highest among pregnant women aged 18–25 years, 7.5% (Brown et al, 2017).
Time Trends in Childhood Exposure
Using data from the National Poison Data System to investigate accidental cannabis exposures in children <6 years old (Onders et al, 2016), the annual rate of exposures increased from 4.2 per million children in 2000 to 10.4 per million in 2013.
Time Trends in Adolescent Cannabis Use
Among NSDUH participants aged 12–17 years, past-month cannabis users fluctuated between 8.2% in 2002 and 7.4% in 2014 (Azofeifa et al, 2016a, b), with no significant overall increases or decreases. Findings from the MTF student surveys also showed that over the same period, rates of cannabis use fluctuated, with some increases up to around 2010, and either stabilization (twelfth graders) or decreases (eighth and tenth graders) from then until 2016 (The Monitoring the Future study and the University of Michigan, 2016). A study of NSDUH participants aged 12–17 years indicated that between 2002 and 2013, the prevalence of CUD decreased (Grucza et al, 2016b), another indicator that cannabis use/problems has not increased among adolescents in recent years.
Time Trends in Adult Cannabis Use, CUD, and Other Indicators of Cannabis-Related Problems
Between 2002 and 2014, past-month cannabis use in NSDUH participants aged 18–25 years increased from 17.3% in 2002 to 19.6% in 2014, a significant overall increase (Azofeifa et al, 2016b), with most of the increase occurring since 2007. Among NSDUH participants ⩾26 years, past-month use also increased significantly between 2002 and 2014 (Azofeifa et al, 2016b), from 4.0 to 6.6% in 2014. In this age group, the increase started in 2008 (Compton et al, 2016). Increases occurred across gender, region, educational level, and employment status (Azofeifa et al, 2016b), with higher rates in males and unemployed participants. Other indicators of adult cannabis use also showed significant increases in NSDUH participants during this period, including first-time use and daily/near-daily use (Compton et al, 2016). Additional studies of NSDUH data showed greater increases in cannabis use among males, especially those with lower incomes (Carliner et al, 2017). However, although daily or near-daily use increased in the entire sample and higher frequency of use is correlated with the risk for a substance use disorder (Compton et al, 2009), NSDUH data did not show increases in the prevalence of adult CUD (Compton et al, 2016) between 2002 and 2014.
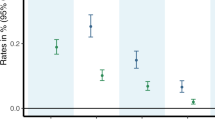
Another source of data on adult trends were surveys conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA): the NESARC, conducted in 2001–2002, and the NESARC-III, conducted in 2012–2013 (see Table 2 for more information about the NIAAA surveys). The past-year prevalence of cannabis use in 2001–2002 and 2012–2013 was 4.1% and 9.5%, respectively, a significant increase (Hasin et al, 2015a, 2017). Significant increases in use were also found across demographic subgroups (sex, age, race/ethnicity, education, marital status, income, urban/rural, and region). The past-year prevalence of DSM-IV CUD was 1.5% in 2001–2002 and 2.9% in 2012–2013, also a significant increase (Hasin et al, 2015a, 2017). Increases in the prevalence of CUD between 2001–2002 and 2012–2013 were also statistically significant across demographic subgroups.
The NSDUH and NESARC surveys were consistent in showing increases in adult cannabis use. However, their findings differed regarding time trends in the prevalence of CUD. Methodological differences between the surveys have been discussed as one possible explanation of the discrepant findings (Compton et al, 2016; Grucza et al, 2016a, c). Another approach to understanding the differences is to determine the consistency of the findings of these surveys with other national indicators of cannabis-related problems or CUD over the same time period (Hasin and Grant, 2016). The NESARC to NESARC-III findings on increases in the prevalence of cannabis use disorders are consistent with these other national trends (see next paragraph), suggesting that the NESARC findings on increased rates of CUD are valid (Hasin and Grant, 2016).
Information on increasing time trends in rates of cannabis-related problems and CUD is available from a wide variety of large-scale data sources, many of them national in scope (Table 3). These show increasing prevalence of ICD-9-CM cannabis use disorders among patients seen in emergency rooms (Zhu and Wu, 2016), hospital inpatients (Charilaou et al, 2017; Shi, 2017), gastroenterology patients (Gubatan et al, 2016), burn treatment patients (Jehle et al, 2015), and in United States veterans treated in the Veterans Health Administration (Bonn-Miller et al, 2012). Of note, the burn treatment patients testing positive for cannabis were younger, less likely to be insured, had larger burns, and required more intensive treatment. Rates of cannabis detected among decedents of fatal motor vehicle (Brady and Li, 2014) and airplane crashes (McKay and Groff, 2016) also increased, as did rates of cannabis detected in roadside surveys of weekend nighttime drivers (Johnson et al, 2012).
Medical and recreational marijuana laws
Medical Marijuana Laws
In 1970, the Federal Drug Enforcement Agency (DEA) defined cannabis as a Schedule 1 substance, meaning no accepted medical use and high abuse potential (U.S. Department of Justice and Drug Enforcement Administration, 2017). However, since 1996, when California passed the first state medical marijuana law (MML), a total of 29 states and the District of Columbia have passed laws legalizing the use of marijuana for medical purposes (Figure 2). State MMLs share the common feature that they permit legal use of cannabis to treat medical conditions if the user has obtained medical authorization. However, the specific provisions of MMLs vary considerably (Pacula et al, 2015), eg, regarding the range and specificity of the permitted medical conditions, the provisions for distribution through dispensaries, permitted amounts per patient, and so on. The restrictiveness or ‘medicalization (Williams et al, 2016, 2017)’ of these laws varies as well. Table 4 shows aspects of MMLs that vary from state to state. All of these have the potential to modify the effects of MMLs, although challenges to research on the effects of these variations arise in their measures because of the between-state variability in the quality and quantity of available data.
US states, medical and recreational marijuana laws.
Concerns about MMLs include their potential to increase problematic use of cannabis in the general population, hypothesized to occur through several mechanisms, including reduced perceived harmfulness and normalization of use (Pacula et al, 2015; Wen et al, 2015). Additional posited mechanisms include greater access via dispensaries and home or caregiver cultivation. Greater access increases availability, and can also normalize the idea of use and reduce perceived harm (Pacula et al, 2015; Wen et al, 2015). Table 5 shows that the percentage of Americans living in MML states has increased over time. Because early and later MMLs were passed in differing national normative contexts, their effects may vary over time (Hasin et al, 2017).
Before 2009, the discrepancy between the Federal and state positions meant that individuals using marijuana as specified by state MMLs were still vulnerable to federal prosecution. However, in 2009, the US Attorney General issued a memorandum instructing federal prosecutors not to prioritize prosecution of individuals compliant with state MMLs (Ogden, 2009). This gave more flexibility to MML states (Cambron et al, 2017), with particular impact in Colorado (Davis et al, 2016b; Hasin et al, 2017; Salomonsen-Sautel et al, 2014; Schuermeyer et al, 2014) and California (Hasin et al, 2017), where dispensaries proliferated. Subsequent memorandums in 2011 and 2013 further clarified these policies by indicating which areas of marijuana law enforcement were still of Federal interest (eg, limiting use among minors; Price 2014).
In states with unrestrictive MMLs (generally states with earlier-passed MMLs), the possibility that medical authorizations were obtained for recreational use is supported by the greater similarity of some medical users to recreational users than to a medically ill population (Harris et al, 2000; Haug et al, 2017; Reinarman et al, 2011; Walsh et al, 2013), and because many medical users used illicit cannabis before their medical authorization (O'Connell and Bou-Matar, 2007). However, two reports on medical marijuana users among adult NSDUH participants provide more representative information. The first (Lin et al, 2016), reporting only on users from MML states, indicated that among past-year cannabis users, 17% used cannabis medically. Medical and recreational users did not differ on race, education, depression, or cannabis use disorders, but medical users were more likely to report poor health and fewer substance use disorders. The second (Compton et al, 2017) reported on all medical cannabis users in all states, as some could use cannabis for medical purposes even if they did not live in a MML state. In this study, 9.8% of all cannabis users were medical users. Their medical use was associated with poor self-reported health, disability, older age, and older age at initiation of cannabis use. Of all medical users, 21.2% lived in states with no MMLs, suggesting that either physicians in non-MML states also recommend medical marijuana use to some patients, or some patients in non-MML states were self-medicating problems with cannabis.
Recreational Marijuana Laws
In 2012, Colorado and Washington became the first states to pass recreational marijuana laws (RMLs). Since then (Figure 2), 6 additional states passed RMLs: Alaska and Oregon (2014), and California, Nevada, Massachusetts, and Maine (2016). Consequently, 21% of the US population now lives in states where recreational use is legal. All eight of the states that passed recreational marijuana laws previously had MML.
RMLs permit legal sale and use of cannabis without the need for medical justification or authorization. Such laws may reduce discriminatory arrests of disadvantaged minorities because of biased enforcement of criminal cannabis statutes (Hall and Lynskey, 2016; Palamar et al, 2014), and satisfy public desire for legal cannabis use. Furthermore, RMLs have the potential to create business opportunities, jobs, and tax revenues (Forbes, 2017; McGinty et al, 2016, 2017a), as exemplified by Colorado (Fortune, 2016) and Washington (The Washington Times, 2016), where cannabis is now a billion-dollar-a-year business (Wang et al, 2017). However, RMLs are likely to increase availability, advertising, and accepting attitudes toward cannabis use, all of which may enlarge the population of cannabis users, increase the rates of adverse health or psychosocial consequences, and have unintended effects of the use of other substances (Volkow et al, 2014). In addition, RMLs are likely to reduce the price of cannabis (Hall and Lynskey, 2016) that may also increase its appeal. Because RMLs are recent, little is known about their impact on public health. Recognizing this, the National Institute on Drug Abuse has made studies of state marijuana laws a priority (National Institute on Drug Abuse, 2015).
Relationship of marijuana laws to trends in use and consequences
Marijuana Laws and Trends in Cannabis Potency
Among samples of illegal cannabis seized by law enforcement between 1990 and 2010 (Sevigny et al, 2014) analyzed by state, the mean THC potency was 9.1% in states that eventually passed MMLs and 5.6% in other states. Initial models of state effects on potency found a MML effect on subsequent cannabis potency, but this effect was no longer significant after controlling for a multitude of potential confounders. However, in states with legally operating dispensaries, the MML effect remained significant, increasing THC potency of ∼1%.
As states increasingly pass recreational marijuana laws, the THC potency of illicit cannabis in MML states may no longer be such a salient issue. In Washington (RML passed in 2012), the average THC potency of marijuana flower for one Seattle-based retailer in 2015 was 21.2% (Washington State Marijuana Impact Report, 2016). In Colorado, where THC potency of legally marketed cannabis can range considerably, some strains have THC potencies of 28–32% (CNN, 2016). In 2016, Colorado legislators attempted to limit THC content of marketed cannabis to 16%, but this attempt failed, indicating local support for continued marketing of stronger forms of cannabis. Furthermore, although smoking remains the most common route of administration (Hall et al, 2016), newly popular routes of administration offer even higher THC doses (Hall et al, 2016). These include edibles, vaping (inhaled vapor of heated e-liquids analogous to e-cigarettes), and dabbing (inhaled vapor from heating highly concentrated forms of cannabis or hashish). The health effects of these methods are not yet known, but warrant monitoring (National Institute on Drug Abuse, 2017).
Marijuana Laws and Trends in Prenatal Exposure
No published studies were found on differences in rates of cannabis use by MML or RML state status. Unpublished results from one study (Brown et al, 2017) showed that in MML and non-MML states, rates of cannabis use in pregnant women did not differ significantly.
Marijuana Laws and Trends in Childhood Exposure
A study of 2005–2011 calls to poison centers for unintentional pediatric cannabis exposures showed little increase in states with no MML, an increase of 11.5% in states passing MMLs in 2005–2011, and an increase of 30.3% in states passing MMLs before 2005 (Wang et al, 2014). In this study, among children unintentionally exposed to cannabis, those in states with MMLs passed before 2005 were more likely to be evaluated in a health-care facility, experience major or moderate effects, and be admitted to critical care units (Wang et al, 2014). Another study found that calls to Colorado poison control centers for unintentional pediatric cannabis exposures between 2009 and 2015 (when medical dispensaries proliferated and recreational use was legalized) increased 34% annually, significantly greater than the 19% increase in cannabis-related calls received by poison control centers in other states (Wang et al, 2016a). A further breakdown of Colorado poison center calls (Wang et al, 2017) showed significant increases in those aged 0–8 and 9–17 years after liberalization of medical marijuana laws in 2010, and an even further increase in these ages after enactment of legalized recreational use in 2014. Thus, liberalization of marijuana laws appears related to increases in childhood nonfatal cannabis exposures.
Marijuana Laws and Trends in Adolescent Cannabis Use
Because of the potential harms of early adolescent cannabis use, concerns were raised that MMLs could increase adolescent use by conveying a message about acceptability or lack of negative health consequences, even if MML implementation was delayed or narrowly defined (Hasin et al, 2015b). A study using cross-sectional national data showed that adolescent cannabis use was more prevalent in MML states (Wall et al, 2011). However, cross-sectional associations do not necessarily indicate a causal relationship, as states with MMLs could have had higher rates of use before MML passage (ie, reverse causation). Although randomized assignment of MMLs to states would provide clarity about the direction of effect, such assignment is clearly not possible. Therefore, studies used difference-in-difference (DiD) tests to examine differences in rates in states before and after MML passage compared with contemporaneous differences during the same years in non-MML states (Angrist and Krueger, 1999; Angrist and Pischke, 2009; Hunt and Miles, 2015; Imbens and Wooldridge, 2009). These studies also controlled for many state- and individual-level confounders to sharpen conclusions on causality. Studies used MTF data from 1991 to 2014 (Hasin et al, 2015b), NSDUH data from 2002 to 2012 (Wen et al, 2015), and several other large databases (Sarvet et al, under review-a). Of the 17 large surveys using DiD methods spanning different states, periods, and specifications, 16 indicated no MML effects on adolescent use (Anderson et al, 2015; Choi, 2014; Hasin et al, 2015b; Keyes et al, 2016; Martins et al, 2016; Pacula et al, 2015; Sarvet et al, under review-a; Smart, 2015; Wen et al, 2015). Despite differences in methodology, the findings were very consistent: post-MML adolescent cannabis use did not increase compared with pre-MML levels and to national trends in non-MML states during the corresponding years. In MTF data, perceived harmfulness of marijuana use decreased and marijuana use increased following legalization of recreational marijuana use in Washington State in 2012 whereas, in contrast Colorado did not exhibit any differential change in perceived harmfulness or past-month adolescent marijuana use following legalization (Cerda et al, 2017).
Marijuana Laws and Trends in Adult Cannabis Use and Cannabis Use Disorders
Fewer studies are available on the relationship of MMLs to adult cannabis outcomes. A cross-sectional study using national 2004–2005 data showed an association between living in a MML state with adult cannabis use and cannabis disorders (Cerda et al, 2012). However, this design also did not address causality. DiD tests were therefore used to examine adult outcomes. A 15–20% post-MML increase occurred in marijuana possession arrests in major cities (Chu, 2014), and a 20% post-MML increase occurred in first-time adult marijuana admissions (Chu, 2014). Using NSDUH 2004–2013 data (Table 2) at a point when 10 states had passed MMLs, DiD tests indicated significant post-MML increases for cannabis use, daily or near-daily use, and (with 1- and 2-year time lags) CUD (Wen et al, 2015), an effect that remained for cannabis use among adults aged ⩾26 years in a reanalysis (Martins et al, 2016). Using DiD tests and three NIAAA surveys from 1991–1992 to 2012–2013 (Table 2) to compare 15 MML states with other states, post-MML effects were found on adult cannabis use and CUD (Hasin et al, 2017). Between 1991–1992 and 2001–2002, rates of adult use and CUD generally decreased, but in contrast, rates of adult use and CUD increased in early-MML states. Between 2001–2002 and 2012–2013, rates generally increased, with greater increases in late-MML than non-MML states. During this later time, California and Colorado showed marked increases, consistent with the proliferation of dispensaries in these two states after the 2009 Department of Justice Memo. Thus, although the research base is not extensive, existing studies are consistent in showing post-MML increases in adult cannabis-related outcomes.
Recreational Marijuana Laws
Aside from Cerda et al (2017) and Wang et al (2017) who suggest that RMLs may have further effects on child exposures or adolescent use in states that already have MMLs, little is known about the effects of recreational marijuana laws on cannabis use, or cannabis-related consequences. To our knowledge, no studies have yet been published on the relationship of RML to adult outcomes, an area that is clearly in need of studies as data become available.
Epidemiologic evidence on mmls and cannabis used as a substitute for opioids or alcohol
Cannabis to Treat Pain and as a Substitute for Opioids
Chronic pain is common in U.S. adults (Hardt et al, 2008; Institute of Medicine, 2011; Nahin, 2015; Tsang et al, 2008). Opioids are important to treat acute pain, but are also widely prescribed for chronic pain (Levy et al, 2015; Volkow and McLellan, 2016) with inconsistent clinical benefits (Chou et al, 2014) and serious risks (Chou et al, 2014; Volkow and McLellan, 2016), eg, physical dependence, addiction, transition to heroin, and overdose (Okie, 2010; Paulozzi et al, 2012; Rudd et al, 2016). Therefore, despite problems associated with medical marijuana (cognitive/motor impairments (Volkow et al, 2016), side effects (Whiting et al, 2015), no standard product formulations (Thomas and Pollard, 2016)), its advantages (analgesia (Whiting et al, 2015), lack of fatal overdose (Hall, 2017), or transition to heroin) have led to professional calls to substitute medical marijuana for opioids (Choo et al, 2016), although this debate continues (Saxon and Browne, 2014). Many medical marijuana patients use it for pain relief, some as a partial or complete substitute for opioids (Boehnke et al, 2016; Davis et al, 2016a; Lucas and Walsh, 2017; Lucas et al, 2016; Nunberg et al, 2011; Piper et al, 2017; Reinarman et al, 2011), and others continuing to use or abuse prescription opioids. In an online convenience sample, participants in both MML and non-MML states used cannabis for pain (Corroon et al, 2017). Thus, studies of adult substitution/complementarity of cannabis and opioids are needed, including whether this occurs more in MML and RML states.
Several ecological studies of MML and rates of opioid outcomes have been conducted, based on the premise that if MMLs provide marijuana to those who need it, opioid use/misuse will be reduced. The studies found that MMLs led to lower rates of opioid prescriptions in Medicaid (Bradford and Bradford, 2017) and Medicare Part D (Bradford and Bradford, 2016a). MMLs also led to lower rates of opioid overdoses (Bachhuber et al, 2014; Pardo, 2016), although according to Bachhuber et al (2014) significance was lost after controlling for state-specific linear time trends that adjusted for differential factors changing linearly over the study period (eg, hard-to-measure attitudes or cultural changes). MMLs were also associated with decreased hospitalization for OUD (Powell et al, 2015; Shi, 2017) and opioids detected in fatally injured drivers (Kim et al, 2016) (but of all ages studied, only in those aged 21–40 years). In the single individual-level study (NSDUH 2004–2012), MML was unrelated to non-medical opioid or heroin use (Wen et al, 2015). Studies are thus largely but not fully consistent. The field concurs that more individual-level studies of MML and opioids are needed, controlling for important individual and state-level covariates (Bradford and Bradford, 2016b, 2017; Finney et al, 2015; Hall and Lynskey, 2016). Opioid outcomes should include medical use, ie, prescriptions; benzodiazepine co-prescriptions (shown repeatedly (Garg et al, 2017; Hawkins et al, 2013; Hwang et al, 2016; Sun et al, 2017) to be very risky for overdose, and common in some groups, eg, veterans (Hawkins et al, 2015)); frequent non-medical use; and consequences of medical or nonmedical use: OUD, DUI, overdose. Studies should include state-level demographic characteristics, MML provisions, and opioid policies. Studies should also determine whether MML effects remain after controlling for important individual demographic and clinical characteristics, eg, pain or pain-related medical conditions, and other substance and psychiatric disorders (Hayes and Brown, 2014).
Adults and Alcohol
Some medical marijuana clients substitute cannabis for alcohol (Lucas and Walsh, 2017; Lucas et al, 2016; Nunberg et al, 2011; Reiman, 2009; Reinarman et al, 2011). In studies that used data from 1990 to 2010, passage of MMLs was followed by decreased alcohol-related traffic fatalities (Anderson et al, 2013) and binge drinking (Anderson et al, 2013), suggesting that cannabis was substituted for alcohol in these states. However, in NSDUH adults surveyed between 2004 and 2012, passage of MMLs was followed by increased binge drinking (Anderson et al, 2013; Guttmannova et al, 2016; Wen et al, 2015). An unpublished economics report showed that passage of MML was followed by increases in any drinking and alcohol-related treatment admissions, but only if the MMLs permitted dispensaries (Pacula et al, 2013). A state-level study using data from 1985 to 2014 showed that passage of MMLs was followed by decreased overall US traffic fatalities (Santaella-Tenorio et al, 2017), particularly in ages 25–44 years, but only in 7 states, and therefore the results were interpreted as indicating heterogeneous effects. Thus, findings on MMLs, alcohol, and cannabis have been inconsistent (Guttmannova et al, 2016), perhaps because of analyses of different outcomes and state-level control variables in the different studies (Guttmannova et al, 2016), leading to the need for further exploration in designs that could clarify the sources of inconsistent results.
Speculation about RML effects largely assume that substituting cannabis for alcohol will be better for public health (Anderson and Rees, 2014; Carnevale et al, 2017). Some experts have assumed that RMLs will lead to substitution of cannabis for alcohol (Anderson and Rees, 2014; Kilmer, 2017), whereas others have been less sure (Edwards, 1974; Hall and Lynskey, 2016; Hawken et al, 2013; Pacula and Sevigny, 2014). Whether cannabis will actually be substituted for alcohol after passage of RMLs, and whether the effects of any such substitution on public health will be positive, negative, or neutral is currently unknown.
Epidemiologic evidence on marijuana laws and cannabis used as a substitute for psychiatric medication
Personal and anecdotal testimonies suggest that cannabis (medical or otherwise) is effective in treating symptoms of depression and anxiety (Broadly, 2016; Grass City, 2010). Consistent with this, surveys of medical marijuana patients show that many of them use marijuana to treat these symptoms (Bohnert et al, 2014; Bonn-Miller et al, 2014; Harris et al, 2000; Nunberg et al, 2011; Piper et al, 2017; Reinarman et al, 2011; Walsh et al, 2017). Although theoretical reasons suggest that synthetic oral cannabinoids may be helpful for some aspects of PTSD (Haney and Evins, 2016), scientific reviews (National Academies of Sciences, 2017; Walsh et al, 2017; Whiting et al, 2015) of studies to date find no evidence for the efficacy of cannabinoids in the treatment of depression or anxiety disorders. In addition, prospective studies show adverse cannabis effects on the course of depression (Bahorik et al, 2017) and PTSD (Wilkinson et al, 2015). When medical marijuana clients are asked about actual symptom relief, less than half report such relief (Bonn-Miller et al, 2014); other medical marijuana clients (Swift et al, 2005) report return of anxiety symptoms on cessation of use, suggesting the symptoms might be due to cannabis withdrawal (Walsh et al, 2017). Because many cannabis withdrawal criteria are depression/anxiety symptoms (American Psychiatric Association, 2013) (Table 1), regular users may seek cannabis to obtain short-term symptom relief, unaware that this could perpetuate a longer-term withdrawal problem. Nevertheless, medical marijuana is authorized for PTSD in 21 states, and in many others, permitted conditions are vague enough that use for depression or anxiety may also be authorized. In nationally representative 2004–2005 data, participants with DSM-IV depressive or anxiety disorders were more likely to self-medicate their symptoms with cannabis if they lived in MML than non-MML states (Sarvet et al, under review-b). However, a more recent survey of a convenience sample suggested that self-medication of depression or anxiety with cannabis is equally common in MML and non-MML states (Corroon et al, 2017). Without recent data from a representative sample, whether the more recent convenience survey reflects a change or a biased result due to the sampling method remains unknown.
In data on all fee-for-service Medicaid prescriptions from 2007 to 2014, antidepressant and anti-anxiety prescriptions were lower in states with MMLs than in other states (Bradford and Bradford, 2017). In 2010–2013 data on prescriptions filled by Medicare Part D enrollees, antidepressant prescriptions fell significantly in MML states once a medical marijuana law was implemented (Bradford and Bradford, 2016a). If cannabis/cannabinoid products were effective treatments for depression or anxiety disorders, substituting cannabis for FDA-approved medication would be a positive MML result (eg, by reducing medical costs (Bradford and Bradford, 2016b)). As evidence on efficacy suggests otherwise (National Academies of Sciences, 2017; Walsh et al, 2017; Whiting et al, 2015), and as marijuana use may be due to confusion between cannabis withdrawal and depressive/anxiety disorders (Swift et al, 2005), such shifts in clinical care would be an adverse MML outcome. However, to understand the effects of MML and RML on use of antidepressants and antianxiety medication, large-scale studies using individual-level data (Bradford and Bradford, 2017) are needed that can include relevant state socioeconomic and policy variables, and individual-level demographic and clinical covariates.
Implications and future research directions
The implications of changing laws, attitudes, and prevalence of cannabis use have implications for clinicians, policy makers, and the public. All should be aware that despite a lack of risk for fatal overdose or transition to heroin, both of which are serious risks for prescription opioids, cannabis is not a harmless substance, and use can involve impairments, addiction, and risks for serious consequences. In NESARC 2001–2002 data, 6.4% of those with current DSM-IV cannabis abuse and 18.1% of those with current DSM-IV cannabis dependence received any kind of intervention for drug use problems (Stinson et al, 2006) and of those with lifetime DSM-IV cannabis abuse or dependence, 9.8 and 34.7% received any type of intervention. Thus, drug treatment among those with cannabis use disorders was rare, and whether the treatment focused on cannabis use disorders is unknown. NESARC-III 2012–2013 data showed that among those with DSM-5 current and lifetime cannabis use disorders, 7.2 and 13.7% received any type of intervention specifically for cannabis problems (Hasin et al, 2016). Furthermore, despite the clear increases in adult cannabis use and related problems in the general population, the proportion of patients in substance abuse treatment whose primary substance was cannabis was 16% in 2003 and 17% in 2013 (Substance Abuse and Mental Health Services Administration and Center for Behavioral Health Statistics and Quality, 2015), showing no increase. Thus, cannabis use disorders remain seriously undertreated in the US general population.
Mental health clinicians, especially those treating younger patients or patients with affective or anxiety symptoms, should consider screening their patients for cannabis use patterns and cannabis use disorder criteria to determine whether a disorder is present, or explore with patients whether a cannabis use/withdrawal cycle may be perpetuating depressive or anxiety symptoms. Although patients may not initially be receptive to the idea that cannabis is causing or contributing to their symptoms or problems rather than alleviating them, continued discussion and some monitoring may help in this regard. Health policy makers, for example, commissioners of state or city mental health or substance abuse services should also be aware of the national trends toward increasing use and consequences, and encourage system-wide awareness of current information on cannabis use and its consequences across all professional levels of service providers. State legislators, when considering passage or modifications of medical or recreational marijuana laws, should consider increases in the occurrence of potential adverse consequences of increasingly widespread use, and design state policies for distributing, advertising, and taxing cannabis with these risks in mind. Finally, the public should be made aware of the risks as well. Public education campaigns based on exaggerated scare tactics are unlikely to be successful. However, public education efforts can be effective in such areas as reduced rates of drunk-driving fatal accidents (Niederdeppe et al, 2017a). Well-designed, evidence-based programs may change public attitudes in a positive, more health-promoting direction on many health policy issues involving substance use (Bachhuber et al, 2015; Niederdeppe et al, 2017b).
This review has identified a number of areas needing further research. The following are open areas of research that could be investigated using epidemiologic designs.
-
1
Studies on the relationship of medical and recreational marijuana laws to indicators of driving under the influence of cannabis, or of other substances.
-
2
Studies of postnatal outcomes among women using marijuana but not other substances while pregnant that may be more possible now than in previous years if women who would not consider drinking alcohol or smoking cigarettes see marijuana as a harmless way to treat pregnancy-related symptoms (The New York Times, 2017).
-
3
Long-term comparisons of cognitive functioning in cannabis users and nonusers, with observation begun in childhood, before cannabis exposure.
-
4
Studies of the relationship between cannabis withdrawal symptoms, diagnoses of depressive and anxiety disorders, self-medication with cannabis, and utilization of psychiatric medication.
-
5
Studies examining the effectiveness of various interventions aimed at increasing public awareness of cannabis risks and their likelihood.
-
6
Continued monitoring of time trends in cannabis use and consequences, overall and by demographic characteristics.
-
7
Continued studies of the relationship of MML to cannabis use and consequences, and to the use of and consequences of other substances, including opioids.
-
8
Studies of the relationship of RML to cannabis use and consequences, and to the use of and consequences of other substances, including opioids.
In the past 10 years, much has been learned about cannabis, its use, and its consequences. However, in this time of rapidly changing marijuana laws and attitudes, much remains to be learned in order to advance public health and to guide personal and societal decisions regarding the use of cannabis.
Funding and disclosure
The author declares no conflict of interest.
References
Agrawal A, Budney AJ, Lynskey MT (2012). The co-occurring use and misuse of cannabis and tobacco: a review. Addiction 107: 1221–1233.
Agrawal A, Lynskey MT (2006). The genetic epidemiology of cannabis use, abuse and dependence. Addiction 101: 801–812.
Agrawal A, Lynskey MT (2007). Does gender contribute to heterogeneity in criteria for cannabis abuse and dependence? Results from the national epidemiological survey on alcohol and related conditions. Drug Alcohol Depend 88: 300–307.
Agrawal A, Lynskey MT (2014). Cannabis controversies: how genetics can inform the study of comorbidity. Addiction 109: 360–370.
Agrawal A, Neale MC, Prescott CA, Kendler KS (2004). A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med 34: 1227–1237.
Agrawal A, Pergadia ML, Lynskey MT (2008). Is there evidence for symptoms of cannabis withdrawal in the national epidemiologic survey of alcohol and related conditions? Am J Addict 17: 199–208.
Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J et al (2012). Quantifying the clinical significance of cannabis withdrawal. PLoS ONE 7: e44864.
American College of Obstetricians, Gynecologists Committee on Obstetric Practice (2015). Committee Opinion No. 637: Marijuana Use During Pregnancy and Lactation. Obstet Gynecol 126: 234–238.
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Association: Washington, DC.
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association: Arlington, VA.
Anderson DM, Hansen B, Rees DI (2013). Medical marijuana laws, traffic fatalities, and alcohol consumption. J Law Econ 56: 333–369.
Anderson DM, Hansen B, Rees DI (2015). Medical marijuana laws and teen marijuana use. Am Law Econ Rev 17: 495–528.
Anderson DM, Rees DI (2014). The legalization of recreational marijuana: how likely is the worst-case scenario? J Policy Anal Manage 33: 221–232.
Angrist J, Krueger AB (1999) Empirical strategies in labor economics. In: Handbook of Labor Economics Vol. 3A. Elsevier: Amsterdam, The Netherlands, Vol. 3A.
Angrist JD, Pischke JS (2009) Mostly Harmless Econometrics: An Empiricist's Companion. Princeton University Press: Princeton.
Anthony JC, Warner LA, Kessler RC (1994). Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol 2: 244–268.
Asbridge M, Hayden JA, Cartwright JL (2012). Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ 344: e536.
Azofeifa A, Mattson ME, Grant A (2016a). Monitoring marijuana use in the United States: challenges in an evolving environment. JAMA 316: 1765–1766.
Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R (2016b). national estimates of marijuana use and related indicators - National Survey on Drug Use and Health, United States, 2002-2014. MMWR Surveill Summ 65: 1–28.
Babor T Alcohol: No Ordinary Commodity. Oxford University Press: New York, 2010.
Bachhuber MA, McGinty EE, Kennedy-Hendricks A, Niederdeppe J, Barry CL (2015). Messaging to increase public support for naloxone distribution policies in the United States: results from a randomized survey experiment. PLoS ONE 10: e0130050.
Bachhuber MA, Saloner B, Cunningham CO, Barry CL (2014). Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med 174: 1668–1673.
Bachman JG, Johnson LD, O'Malley PM (1998). Explaining recent increases in students' marijuana use: impacts of perceived risks and disapproval, 1976 through 1996. Am J Public Health 88: 887–892.
Bachman JG, Johnston LD, O’Malley PM, Humphrey RH (1988). Explaining the recent decline in marijuana use: Differentiating the effects of perceived risks, disapproval, and general lifestyle factors. J Health Soc Behav 29: 92–112.
Bahorik AL, Leibowitz A, Sterling SA, Travis A, Weisner C, Satre DD (2017). Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J Affect Disord 213: 168–171.
Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S et al (2013). Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS ONE 8: e55821.
Baumann AF, Scheinbaum C (2016). Weed Rosin Is Changing the Way We Get High. Bloomberg Businessweek. Available at https://www.bloomberg.com/news/articles/2016-03-23/weed-rosin-is-changing-the-way-we-get-high. Accessed 3 July 2017.
Blanco C, Hasin DS, Wall MM, Florez-Salamanca L, Hoertel N, Wang S et al (2016). Cannabis use and risk of psychiatric disorders: prospective evidence from a US National Longitudinal Study. JAMA Psychiatry 73: 388–395.
Boden JM, Fergusson DM, Horwood LJ (2007). Anxiety disorders and suicidal behaviours in adolescence and young adulthood: findings from a longitudinal study. Psychol Med 37: 431–440.
Boehnke KF, Litinas E, Clauw DJ (2016). Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain 17: 739–744.
Bogdan R, Winstone JM, Agrawal A (2016). Genetic and environmental factors associated with cannabis involvement. Curr Addict Rep 3: 199–213.
Bohnert KM, Perron BE, Ashrafioun L, Kleinberg F, Jannausch M, Ilgen MA (2014). Positive posttraumatic stress disorder screens among first-time medical cannabis patients: prevalence and association with other substance use. Addict Behav 39: 1414–1417.
Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA (2014). Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 40: 23–30.
Bonn-Miller MO, Harris AH, Trafton JA (2012). Prevalence of cannabis use disorder diagnoses among veterans in 2002, 2008, and 2009. Psychol Serv 9: 404–416.
Bradford AC, Bradford WD (2016a). Medical marijuana laws reduce prescription medication use in Medicare Part D. Health Aff (Millwood) 35: 1230–1236.
Bradford AC, Bradford WD (2016b). Medical marijuana laws: the authors reply. Health Aff (Millwood) 35: 1937.
Bradford AC, Bradford WD (2017). Medical marijuana laws may be associated with a decline in the number of prescriptions for Medicaid enrollees. Health Aff (Millwood) 36: 945–951.
Brady JE, Li G (2014). Trends in alcohol and other drugs detected in fatally injured drivers in the United States, 1999-2010. Am J Epidemiol 179: 692–699.
Broadly (2016). Why people smoke weed to treat depression. Available at https://broadly.vice.com/en_us/article/bmwwmz/why-people-smoke-weed-to-treat-depression. Accessed 29 June 2017.
Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS (2017). Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002-2014. JAMA 317: 207–209.
Budney AJ, Hughes JR (2006). The cannabis withdrawal syndrome. Curr Opin Psychiatry 19: 233–238.
Budney AJ, Hughes JR, Moore BA, Vandrey R (2004). Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 161: 1967–1977.
Budney AJ, Moore BA, Vandrey RG, Hughes JR (2003). The time course and significance of cannabis withdrawal. J Abnorm Psychol 112: 393–402.
Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B (2007). Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend 86: 22–29.
Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z (2008). Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat 35: 362–368.
Bujarski SJ, Galang JN, Short NA, Trafton JA, Gifford EV, Kimerling R et al (2016). Cannabis use disorder treatment barriers and facilitators among veterans with PTSD. Psychol Addict Behav 30: 73–81.
Calabria B, Degenhardt L, Hall W, Lynskey M (2010). Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev 29: 318–330.
Cambron C, Guttmannova K, Fleming CB (2017). State and national contexts in evaluating cannabis laws: a case study of Washington state. J Drug Issues 47: 74–90.
Carey CE, Agrawal A, Bucholz KK, Hartz SM, Lynskey MT, Nelson EC et al (2016). Associations between polygenic risk for psychiatric disorders and substance involvement. Front Genet 7: 149.
Carliner H, Mauro PM, Brown QL, Shmulewitz D, Rahim-Juwel R, Sarvet AL et al (2017). The widening gender gap in marijuana use prevalence in the U.S. during a period of economic change, 2002-2014. Drug Alcohol Depend 170: 51–58.
Carnevale JT, Kagan R, Murphy PJ, Esrick J (2017). A practical framework for regulating for-profit recreational marijuana in US states: lessons from Colorado and Washington. Int J Drug Policy 42: 71–85.
Carney R, Cotter J, Firth J, Bradshaw T, Yung AR (2017). Cannabis use and symptom severity in individuals at ultra high risk for psychosis: a meta-analysis. Acta Psychiatr Scand 136: 5–15.
Cascini F, Aiello C, Di Tanna G (2012). Increasing delta-9-tetrahydrocannabinol (Delta-9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr Drug Abuse Rev 5: 32–40.
Cerda M, Wall M, Feng T, Keyes KM, Sarvet A, Schulenberg J et al (2017). Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatr 171: 142–149.
Cerda M, Wall M, Keyes KM, Galea S, Hasin D (2012). Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend 120: 22–27.
Charilaou P, Agnihotri K, Garcia P, Badheka A, Frenia D, Yegneswaran B (2017). Trends of cannabis use disorder in the inpatient: 2002 to 2011. Am J Med 130: 678–687 e677.
Chen CY, Storr CL, Anthony JC (2009). Early-onset drug use and risk for drug dependence problems. Addict Behav 34: 319–322.
Choi A (2014) The Impact of Medical Marijuana Laws on Marijuana Use And Other Risky Health Behaviors. Presented at the American Society for Health Economics, Los Angeles, CA.
Choo EK, Feldstein Ewing SW, Lovejoy TI (2016). Opioids out, cannabis in: negotiating the unknowns in patient care for chronic pain. JAMA 316: 1763–1764.
Chou R, Deyo R, Devine B, Hansen R, Sullivan S, Jarvik JG et al (2014). The Effectiveness and Risks of Long-Term Opioid Treatment of Chronic Pain. AHRQ Publication No. 14-E005-EF. Agency for Healthcare Research and Quality: Rockville, MD. Available at www.effectivehealthcare.ahrq.gov/reports/final.cfm Accessed 1 June 2017.
Chu YW (2014). The effects of medical marijuana laws on illegal marijuana use. J Health Econ 38: 43–61.
Chung T, Martin CS, Cornelius JR, Clark DB (2008). Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction 103: 787–799.
CNN (2016). Colorado marijuana's potency getting 'higher'. Available at http://www.cnn.com/2016/10/21/health/colorado-marijuana-potency-above-national-average/index.html?_sm_au_=iVVwFVZDW6Q7kD46 Accessed 2 July 2017.
Compton WM, Han B, Hughes A, Jones CM, Blanco C (2017). Use of marijuana for medical purposes among adults in the United States. JAMA 317: 209–211.
Compton WM, Han B, Jones CM, Blanco C, Hughes A (2016). Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry 3: 954–964.
Compton WM, Saha TD, Conway KP, Grant BF (2009). The role of cannabis use within a dimensional approach to cannabis use disorders. Drug Alcohol Depend 100: 221–227.
Connell CM, Gilreath TD, Aklin WM, Brex RA (2010). Social-ecological influences on patterns of substance use among non-metropolitan high school students. Am J Community Psychol 45: 36–48.
Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC et al (2006). Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict 15: 8–14.
Corbett KK (2001). Susceptibility of youth to tobacco: a social ecological framework for prevention. Respir Physiol 128: 103–118.
Cornelius JR, Chung T, Martin C, Wood DS, Clark DB (2008). Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav 33: 1500–1505.
Corroon JM Jr, Mischley LK, Sexton M (2017). Cannabis as a substitute for prescription drugs - a cross-sectional study. J Pain Res 10: 989–998.
Davis AK, Bonar EE, Ilgen MA, Walton MA, Perron BE, Chermack ST (2016a). Factors associated with having a medical marijuana card among veterans with recent substance use in VA outpatient treatment. Addict Behav 63: 132–136.
Davis JM, Mendelson B, Berkes JJ, Suleta K, Corsi KF, Booth RE (2016b). Public health effects of medical marijuana legalization in Colorado. Am J Prev Med 50: 373–379.
Edwards G (1974). Cannabis and the criteria for legalisation of a currently prohibited recreational drug: groundwork for a debate. Acta Psychiatr Scand Suppl 251: 1–62.
El Marroun H, Tiemeier H, Franken IH, Jaddoe VW, van der Lugt A, Verhulst FC et al (2016). Prenatal cannabis and tobacco exposure in relation to brain morphology: a prospective neuroimaging study in young children. Biol Psychiatry 79: 971–979.
Elkashef A, Vocci F, Huestis M, Haney M, Budney A, Gruber A et al (2008). Marijuana neurobiology and treatment. Subst Abus 29: 17–29.
ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC (2016). Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry 79: 613–619.
Englund A, Freeman TP, Murray RM, McGuire P (2017). Can we make cannabis safer? Lancet Psychiatry 4: 643–648.
Fergusson DM, Boden JM (2008). Cannabis use and later life outcomes. Addiction 103: 969–976 discussion 977-968.
Fergusson DM, Boden JM, Horwood LJ (2015). Psychosocial sequelae of cannabis use and implications for policy: findings from the Christchurch Health and Development Study. Soc Psychiatry Psychiatr Epidemiol 50: 1317–1326.
Finney JW, Humphreys K, Harris AH (2015). What ecologic analyses cannot tell us about medical marijuana legalization and opioid pain medication mortality. JAMA Intern Med 175: 655–656.
Forbes (2017). $1 Billion in Marijuana Taxes Is Addictive to State Governors. Available at https://www.forbes.com/sites/debraborchardt/2017/04/11/1-billion-in-marijuana-taxes-is-addicting-to-state-governors/#5b20042d2c3b Accessed 30 June 2017.
Fortune (2016). Colorado Topped $1 Billion in Legal Marijuana Sales in 2016. Available at http://www.fortune.com/2016/12/13/colorado-billion-legal-marijuana-sales/?_sm_au_=iVVwFVZDW6Q7kD46 Accessed 30 June 2017.
Gage SH, Hickman M, Zammit S (2016). Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry 79: 549–556.
Garg RK, Fulton-Kehoe D, Franklin GM (2017). Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Med Care 55: 661–668.
Goldstein RZ, Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12: 652–669.
Grant BF (1996). DSM-IV, DSM-III-R, and ICD-10 alcohol and drug abuse/harmful use and dependence, United States, 1992: a nosological comparison. Alcohol Clin Exp Res 20: 1481–1488.
Grass City (2010). Marijuana cured my depression. Available at https://forum.grasscity.com/threads/marijuana-cured-my-depression.683017/?_sm_au_=iVVN7Zq5H3sKjdZq. Accessed 29 June 2017.
Greene MC, Kelly JF (2014). The prevalence of cannabis withdrawal and its influence on adolescents' treatment response and outcomes: a 12-month prospective investigation. J Addict Med 8: 359–367.
Grucza RA, Agrawal A, Bierut LJ (2016a). NESARC findings on increased prevalence of marijuana use disorders-reply: consistent with other sources of information. JAMA Psychiatry 73: 532–533.
Grucza RA, Agrawal A, Krauss MJ, Bongu J, Plunk AD, Cavazos-Rehg PA et al (2016b). declining prevalence of marijuana use disorders among adolescents in the United States, 2002 to 2013. J Am Acad Child Adolesc Psychiatry 55: 487–494 e486.
Grucza RA, Agrawal A, Krauss MJ, Cavazos-Rehg PA, Bierut LJ (2016c). Recent trends in the prevalence of marijuana use and associated disorders in the United States. JAMA Psychiatry 73: 300–301.
Gruenewald PJ (2011). Regulating availability: how access to alcohol affects drinking and problems in youth and adults. Alcohol Res Health 34: 248–256.
Gruenewald PJ, Remer LG, LaScala EA (2014). Testing a social ecological model of alcohol use: the California 50-city study. Addiction 109: 736–745.
Gubatan J, Staller K, Barshop K, Kuo B (2016). Cannabis abuse is increasing and associated with increased emergency department utilization in gastroenterology patients. Dig Dis Sci 61: 1844–1852.
Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C et al (2016). Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 6: e009986.
Guttmannova K, Lee CM, Kilmer JR, Fleming CB, Rhew IC, Kosterman R et al (2016). Impacts of changing marijuana policies on alcohol use in the United States. Alcohol Clin Exp Res 40: 33–46.
Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH et al (2011). Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction 106: 215–224.
Hall W (2017). Alcohol and cannabis: comparing their adverse health effects and regulatory regimes. Int J Drug Policy 42: 57–62.
Hall W, Lynskey M (2016). Evaluating the public health impacts of legalizing recreational cannabis use in the United States. Addiction 111: 1764–1773.
Hall W, Renström M, Poznyak V (2016) The Health and Social Effects of Nonmedical Cannabis Use. World Health Organization: Geneva.
Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD et al (2013). Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry 73: 242–248.
Haney M, Evins AE (2016). Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology 41: 393–401.
Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C et al (2004). Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology 29: 158–170.
Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D (2008). Prevalence of chronic pain in a representative sample in the United States. Pain Med 9: 803–812.
Harris D, Jones RT, Shank R, Nath R, Fernandez E, Goldstein K et al (2000). Self-reported marijuana effects and characteristics of 100 San Francisco medical marijuana club members. J Addict Dis 19: 89–103.
Hartman RL, Huestis MA (2013). Cannabis effects on driving skills. Clin Chem 59: 478–492.
Hasin D, Grant BF, Cottler L, Blaine J, Towle L, Ustun B et al (1997). Nosological comparisons of alcohol and drug diagnoses: a multisite, multi-instrument international study. Drug Alcohol Depend 47: 217–226.
Hasin D, Hatzenbuehler ML, Keyes K, Ogburn E (2006). Substance use disorders: Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and International Classification of Diseases, tenth edition (ICD-10). Addiction 101 (Suppl 1): 59–75.
Hasin DS, Grant B (2016). NESARC findings on increased prevalence of marijuana use disorders-consistent with other sources of information. JAMA Psychiatry 73: 532.
Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM et al (2016). Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: findings from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Am J Psychiatry 173: 588–599.
Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF (2008). Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry 69: 1354–1363.
Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A et al (2013). DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 170: 834–851.
Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H et al (2015a). Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry 72: 1235–1242.
Hasin DS, Sarvet AL, Cerdá M, Keyes KM, Stohl M, Galea S et al (2017). US adult illicit cannabis use, cannabis disorder, and medical marijuana laws: 1991-1992 to 2012-2013. JAMA Psychiatry 74: 579–588.
Hasin DS, Wall M, Keyes KM, Cerda M, Schulenberg J, O'Malley PM et al (2015b). Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry 2: 601–608.
Haug NA, Padula CB, Sottile JE, Vandrey R, Heinz AJ, Bonn-Miller MO (2017). Cannabis use patterns and motives: a comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav 72: 14–20.
Hawken A, Caulkins J, Kilmer B, Kleiman M (2013). Quasi-legal cannabis in Colorado and Washington: local and national implications. Addiction 108: 837–838.
Hawkins EJ, Malte CA, Grossbard J, Saxon AJ, Imel ZE, Kivlahan DR (2013). Comparative safety of benzodiazepines and opioids among veterans affairs patients with posttraumatic stress disorder. J Addict Med 7: 354–362.
Hawkins EJ, Malte CA, Grossbard JR, Saxon AJ (2015). Prevalence and trends of concurrent opioid analgesic and benzodiazepine use among Veterans Affairs patients with post-traumatic stress disorder, 2003-2011. Pain Med 16: 1943–1954.
Hayes MJ, Brown MS (2014). Legalization of medical marijuana and incidence of opioid mortality. JAMA Intern Med 174: 1673–1674.
Hodgson K, Almasy L, Knowles EE, Kent JW Jr, Curran JE, Dyer TD et al (2017). The genetic basis of the comorbidity between cannabis use and major depression. Addiction 112: 113–123.
Hunt PE, Miles J (2015). The Impact of legalizing and regulating weed: issues with study design and emerging findings in the USA. Curr Top Behav Neurosci 34: 173–198.
Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK (2016). Trends in the concomitant prescribing of opioids and benzodiazepines, 2002-2014. Am J Prev Med 51: 151–160.
Imbens GW, Wooldridge JM (2009). Recent developments in the econometrics of program evaluation. J Econ Lit 47: 5–86.
Institute of Medicine (2011) Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press (US): Washington, DC.
Janz NK, Becker MH (1984). The Health Belief Model: a decade later. Health Educ Q 11: 1–47.
Jehle CC Jr, Nazir N, Bhavsar D (2015). The rapidly increasing trend of cannabis use in burn injury. J Burn Care Res 36: e12–e17.
Johnson MB, Kelley-Baker T, Voas RB, Lacey JH (2012). The prevalence of cannabis-involved driving in California. Drug Alcohol Depend 123: 105–109.
Johnston LD, Bachman JG, O'Malley PM (1981) Highlights from Student Drug Use in America, 1975–1980.
Joy JE, Watson SJ, Benson JA (2017) Marijuana and Medicine: Assessing the Science Base. National Academies Press: Washington, DC.
Katz G, Lobel T, Tetelbaum A, Raskin S (2014). Cannabis withdrawal - a new diagnostic category in DSM-5. Isr J Psychiatry Relat Sci 51: 270–275.
Keyes KM, Wall M, Cerda M, Schulenberg J, O'Malley PM, Galea S et al (2016). How does state marijuana policy affect US youth? Medical marijuana laws, marijuana use and perceived harmfulness: 1991-2014. Addiction 111: 2187–2195.
Kilmer B (2017). Recreational cannabis - minimizing the health risks from legalization. N Engl J Med 376: 705–707.
Kim JH, Santaella-Tenorio J, Mauro C, Wrobel J, Cerda M, Keyes KM et al (2016). State medical marijuana laws and the prevalence of opioids detected among fatally injured drivers. Am J Public Health 106: 2032–2037.
Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM (2015). Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol 213: 201 e201–201 e210.
Kouri EM, Pope HG Jr (2000). Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol 8: 483–492.
Kraan T, Velthorst E, Koenders L, Zwaart K, Ising HK, van den Berg D et al (2016). Cannabis use and transition to psychosis in individuals at ultra-high risk: review and meta-analysis. Psychol Med 46: 673–681.
Lee D, Vandrey R, Milman G, Bergamaschi M, Mendu DR, Murray JA et al (2013). Oral fluid/plasma cannabinoid ratios following controlled oral THC and smoked cannabis administration. Anal Bioanal Chem 405: 7269–7279.
Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL et al (2010). Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend 111: 120–127.
Levy B, Paulozzi L, Mack KA, Jones CM (2015). Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med 49: 409–413.
Li MC, Brady JE, DiMaggio CJ, Lusardi AR, Tzong KY, Li G (2012). Marijuana use and motor vehicle crashes. Epidemiol Rev 34: 65–72.
Lichtman AH, Martin BR (2002). Marijuana withdrawal syndrome in the animal model. J Clin Pharmacol 42 (11 Suppl): 20S–27S.
Lin LA, Ilgen MA, Jannausch M, Bohnert KM (2016). Comparing adults who use cannabis medically with those who use recreationally: Results from a national sample. Addict Behav 61: 99–103.
Liu C, Huang Y, Pressley JC (2016). Restraint use and risky driving behaviors across drug types and drug and alcohol combinations for drivers involved in a fatal motor vehicle collision on U.S. roadways. Inj Epidemiol 3: 9.
Lucas P, Walsh Z (2017). Medical cannabis access, use, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int J Drug Policy 42: 30–35.
Lucas P, Walsh Z, Crosby K, Callaway R, Belle-Isle L, Kay R et al (2016). Substituting cannabis for prescription drugs, alcohol and other substances among medical cannabis patients: the impact of contextual factors. Drug Alcohol Rev 35: 326–333.
Marsot A, Audebert C, Attolini L, Lacarelle B, Micallef J, Blin O (2016). Comparison of cannabinoid concentrations in plasma, oral fluid and urine in occasional cannabis smokers after smoking cannabis cigarette. J Pharm Pharm Sci 19: 411–422.
Martinez D, Kim JH, Krystal J, Abi-Dargham A (2007). Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am 17: 539–555.
Martins SS, Mauro CM, Santaella-Tenorio J, Kim JH, Cerda M, Keyes KM et al (2016). State-level medical marijuana laws, marijuana use and perceived availability of marijuana among the general U.S. population. Drug Alcohol Depend 169: 26–32.
McGinty EE, Niederdeppe J, Heley K, Barry CL (2017a). Public perceptions of arguments supporting and opposing recreational marijuana legalization. Prev Med 99: 80–86.
McGinty EE, Samples H, Bandara SN, Saloner B, Bachhuber MA, Barry CL (2016). The emerging public discourse on state legalization of marijuana for recreational use in the US: analysis of news media coverage, 2010-2014. Prev Med 90: 114–120.
McGinty EE, Tung G, Shulman-Laniel J, Hardy R, Rutkow L, Frattaroli S et al (2017b). ignition interlock laws: effects on fatal motor vehicle crashes, 1982-2013. Am J Prev Med 52: 417–423.
McKay MP, Groff L (2016). 23 years of toxicology testing fatally injured pilots: implications for aviation and other modes of transportation. Accid Anal Prev 90: 108–117.
Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS et al (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 109: E2657–E2664.
Milin R, Manion I, Dare G, Walker S (2008). Prospective assessment of cannabis withdrawal in adolescents with cannabis dependence: a pilot study. J Am Acad Child Adolesc Psychiatry 47: 174–178.
The Monitoring the Future study, the University of Michigan (2016). Figures 3 and 4, Marijuana: Trends in annual and daily use in Grades 8, 10 and 12. University of Michigan News Service. Available at: http://www.monitoringthefuture.org/data/16data.html Accessed 22 March 2017.
Musto DF (1991). Opium, cocaine and marijuana in American history. Sci Am 265: 40–47.
Nahin RL (2015). Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 16: 769–780.
National Academies of Sciences, Engineering, and Medicine (2017) The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Press: Washington, DC.
National Institute on Drug Abuse (2015). 2016-2020 NIDA Strategic Plan. Available at https://www.drugabuse.gov/about-nida/2016-2020-nida-strategic-plan Accessed 28 March 2017.
National Institute on Drug Abuse (2017). Is marijuana addictive? Available at https://www.drugabuse.gov/publications/research-reports/marijuana/marijuana-addictive Accessed 2 July 2017.
National Institutes of Health (2015). Adolescent brain cognitive development study. Available at https://addictionresearch.nih.gov/abcd-study Accessed 1 April 2017.
The New York Times (2017). A balm when you're expecting: sometimes pot does the trick. Available at https://www.nytimes.com/2017/02/20/health/marijuana-pregnancy-mothers.html.
Niederdeppe J, Avery R, Miller EN (2017a). Alcohol-control PSAs and drunk-driving fatal accidents in the United States, 1996-2010. Prev Med 99: 320–325.
Niederdeppe J, Kellogg M, Skurka C, Avery RJ (2017b). Market-level exposure to state antismoking media campaigns and public support for tobacco control policy in the United States, 2001-2002. Tob Control.
Nunberg H, Kilmer B, Pacula RL, Burgdorf J (2011). An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal 4: pii: 1.
O'Connell TJ, Bou-Matar CB (2007). Long term marijuana users seeking medical cannabis in California (2001-2007): demographics, social characteristics, patterns of cannabis and other drug use of 4117 applicants. Harm Reduct J 4: 16.
Ogden DW (2009). Memorandum for Selected United States Attorneys on Investigations and Prosecutions in States Authorizing the Medical Use of Marijuana. The United States Department of Justice Archives. Available at. https://www.justice.gov/archives/opa/blog/memorandum-selected-united-state-attorneys-investigations-and-prosecutions-states. Accessed 20 March 2017.
Okie S (2010). A flood of opioids, a rising tide of deaths. N Engl J Med 363: 1981–1985.
Onders B, Casavant MJ, Spiller HA, Chounthirath T, Smith GA (2016). Marijuana exposure among children younger than six years in the United States. Clin Pediatr (Phila) 55: 428–436.
Pacek LR, Mauro PM, Martins SS (2015). Perceived risk of regular cannabis use in the United States from 2002 to 2012: differences by sex, age, and race/ethnicity. Drug Alcohol Depend 149: 232–244.
Pacula RL, Powell D, Heaton P, Sevigny EL (2013). Assessing the effects of marijuana on marijuana and alcohol use: the devil is in the details. Available at http://www.nber.org/papers/w19302. Accessed 16 May 2016.
Pacula RL, Powell D, Heaton P, Sevigny EL (2015). Assessing the effects of medical marijuana laws on marijuana use: the devil is in the details. J Policy Anal Manage 34: 7–31.
Pacula RL, Sevigny EL (2014). Natural experiments in a complex and dynamic environment: the need for a measured assessment of the evidence. J Policy Anal Manage 33: 232–235.
Palamar JJ, Ompad DC, Petkova E (2014). Correlates of intentions to use cannabis among US high school seniors in the case of cannabis legalization. Int J Drug Policy 25: 424–435.
Pardo B (2016). Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction 112: 1773–1783.
Paulozzi LJ, Kilbourne EM, Shah NG, Nolte KB, Desai HA, Landen MG et al (2012). A history of being prescribed controlled substances and risk of drug overdose death. Pain Med 13: 87–95.
Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN et al (2005). Transdermal nicotine alters some of marihuana's effects in male and female volunteers. Drug Alcohol Depend 79: 211–223.
Piontek D, Kraus L, Bjarnason T, Demetrovics Z, Ramstedt M (2013). Individual and country-level effects of cannabis-related perceptions on cannabis use. A multilevel study among adolescents in 32 European countries. J Adolesc Health 52: 473–479.
Piper BJ, DeKeuster RM, Beals ML, Cobb CM, Burchman CA, Perkinson L et al (2017). Substitution of medical cannabis for pharmaceutical agents for pain, anxiety, and sleep. J Psychopharmacol 31: 569–575.
Powell D, Pacula R, Jacobson M (2015). Do medical marijuana laws reduce addictions and deaths related to pain killers? Available at http://www.nber.org/papers/w21345.
Price ZS (2014). Enforcement discretion and executive duty. Vanderbilt Law Rev 67: 671–769.
Pull CB, Saunders JB, Mavreas V, Cottler LB, Grant BF, Hasin DS et al (1997). Concordance between ICD-10 alcohol and drug use disorder criteria and diagnoses as measured by the AUDADIS-ADR, CIDI and SCAN: results of a cross-national study. Drug Alcohol Depend 47: 207–216.
Rabin RA, George TP (2015). A review of co-morbid tobacco and cannabis use disorders: possible mechanisms to explain high rates of co-use. Am J Addict 24: 105–116.
Ramaekers JG, Berghaus G, van Laar M, Drummer OH (2004). Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend 73: 109–119.
Reiman A (2009). Cannabis as a substitute for alcohol and other drugs. Harm Reduct J 6: 35.
Reinarman C, Nunberg H, Lanthier F, Heddleston T (2011). Who are medical marijuana patients? Population characteristics from nine California assessment clinics. J Psychoactive Drugs 43: 128–135.
Rogeberg O, Elvik R (2016). The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction 111: 1348–1359.
Rounsaville BJ, Bryant K, Babor T, Kranzler H, Kadden R (1993). Cross system agreement for substance use disorders: DSM-III-R, DSM-IV and ICD-10. Addiction 88: 337–348.
Rudd RA, Aleshire N, Zibbell JE, Gladden RM (2016). Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep 64: 1378–1382.
Salomonsen-Sautel S, Min SJ, Sakai JT, Thurstone C, Hopfer C (2014). Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend 140: 137–144.
San Nicolas AC, Lemos NP (2015). Toxicology findings in cases of hanging in the city and county of San Francisco over the 3-year period from 2011 to 2013. Forensic Sci Int 255: 146–155.
Santaella-Tenorio J, Mauro CM, Wall MM, Kim JH, Cerda M, Keyes KM et al (2017). US traffic fatalities, 1985-2014, and their relationship to medical marijuana laws. Am J Public Health 107: 336–342.
Sarvet A, Wall M, Fink DS, Greene E, Le A, Boustead AE et al (under review-a). Medical marijuana laws and adolescent marijuana use: a systematic review.
Sarvet AL, Wall MM, Keyes KM, Olfson M, Cerda M, Hasin DS (under review-b). Self-medication of mood and anxiety disorders with marijuana: higher in states with medical marijuana laws.
Saunders JB (2017). Substance use and addictive disorders in DSM-5 and ICD 10 and the draft ICD 11. Curr Opin Psychiatry 30: 227–237.
Saxon AJ, Browne KW (2014). Marijuana not ready for prime time as an analgesic. Gen Hosp Psychiatry 36: 4–6.
Schmidt LA, Jacobs LM, Spetz J (2016). Young people's more permissive views about marijuana: local impact of state laws or national trend? Am J Public Health 106: 1498–1503.
Schuermeyer J, Salomonsen-Sautel S, Price RK, Balan S, Thurstone C, Min SJ et al (2014). Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003-11. Drug Alcohol Depend 140: 145–155.
Sevigny EL, Pacula RL, Heaton P (2014). The effects of medical marijuana laws on potency. Int J Drug Policy 25: 308–319.
Sherva R, Wang Q, Kranzler H, Zhao H, Koesterer R, Herman A et al (2016). Genome-wide association study of cannabis dependence severity, novel risk variants, and shared genetic risks. JAMA Psychiatry 73: 472–480.
Shi Y (2017). Medical marijuana policies and hospitalizations related to marijuana and opioid pain reliever. Drug Alcohol Depend 173: 144–150.
Smart R (2015). The Kids Aren't Alright but Older Adults Are Just Fine: Effects of Medical Marijuana Market Growth on Substance Use and Abuse. S. S. R. Network. Available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2574915.
Smolkina M, Morley KI, Rijsdijk F, Agrawal A, Bergin JE, Nelson EC et al (2017). Cannabis and depression: a twin model approach to co-morbidity. Behav Genet 47: 394–404.
Stern TA, Gross AF, Stern TW, Nejad SH, Maldonado JR (2010). Current approaches to the recognition and treatment of alcohol withdrawal and delirium tremens: "old wine in new bottles" or "new wine in old bottles". Prim Care Companion J Clin Psychiatry 12: pii: PCC.10r00991.
Stinson FS, Ruan WJ, Pickering R, Grant BF (2006). Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med 36: 1447–1460.
Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality (2015). Treatment Episode Data Set (TEDS): 2003-2013. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration. Available at https://www.samhsa.gov/data/sites/default/files/2013_Treatment_Episode_Data_Set_National/2013_Treatment_Episode_Data_Set_National.pdf.
Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S (2017). Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 356: j760.
Swaim RC (2003). Individual and school level effects of perceived harm, perceived availability, and community size on marijuana use among 12th-grade students: a random effects model. Prev Sci 4: 89–98.
Swift W, Gates P, Dillon P (2005). Survey of Australians using cannabis for medical purposes. Harm Reduct J 2: 18.
Tashkin D (2015). How beneficial is vaping cannabis to respiratory health compared to smoking? Addiction 110: 1706–1707.
Thomas BF, Pollard GT (2016). Preparation and distribution of cannabis and cannabis-derived dosage formulations for investigational and therapeutic use in the United States. Front Pharmacol 7: 285.
Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC et al (2008). Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 9: 883–891.
U.S. Department of Justice, Drug Enforcement Administration (2017). Controlled substance schedules. Available at: https://www.deadiversion.usdoj.gov/schedules/ Accessed 15 March 2017.
Ustun B, Compton W, Mager D, Babor T, Baiyewu O, Chatterji S et al (1997). WHO Study on the reliability and validity of the alcohol and drug use disorder instruments: overview of methods and results. Drug Alcohol Depend 47: 161–169.
Verweij KJ, Agrawal A, Nat NO, Creemers HE, Huizink AC, Martin NG et al (2013a). A genetic perspective on the proposed inclusion of cannabis withdrawal in DSM-5. Psychol Med 43: 1713–1722.
Verweij KJ, Vinkhuyzen AA, Benyamin B, Lynskey MT, Quaye L, Agrawal A et al (2013b). The genetic aetiology of cannabis use initiation: a meta-analysis of genome-wide association studies and a SNP-based heritability estimation. Addict Biol 18: 846–850.
Volkow ND, Baler RD, Compton WM, Weiss SR (2014). Adverse health effects of marijuana use. N Engl J Med 370: 2219–2227.
Volkow ND, Compton WM, Wargo EM (2017). The risks of marijuana use during pregnancy. JAMA 317: 129–130.
Volkow ND, McLellan AT (2016). Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 374: 1253–1263.
Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R et al (2016). Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry 73: 292–297.
Wall MM, Poh E, Cerda M, Keyes KM, Galea S, Hasin DS (2011). Adolescent marijuana use from 2002 to 2008: higher in states with medical marijuana laws, cause still unclear. Ann Epidemiol 21: 714–716.
Walsh Z, Callaway R, Belle-Isle L, Capler R, Kay R, Lucas P et al (2013). Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int J Drug Policy 24: 511–516.
Walsh Z, Gonzalez R, Crosby K, S Thiessen M, Carroll C, Bonn-Miller MO (2017). Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev 51: 15–29.
Wang GS, Hall K, Vigil D, Banerji S, Monte A, VanDyke M (2017). Marijuana and acute health care contacts in Colorado. Prev Med. pii: S0091-7435(17)30120-2.
Wang GS, Le Lait MC, Deakyne SJ, Bronstein AC, Bajaj L, Roosevelt G (2016a). Unintentional pediatric exposures to marijuana in Colorado, 2009-2015. JAMA Pediatr 170: e160971.
Wang GS, Roosevelt G, Le Lait MC, Martinez EM, Bucher-Bartelson B, Bronstein AC et al (2014). Association of unintentional pediatric exposures with decriminalization of marijuana in the United States. Ann Emerg Med 63: 684–689.
Wang JB, Ramo DE, Lisha NE, Cataldo JK (2016b). Medical marijuana legalization and cigarette and marijuana co-use in adolescents and adults. Drug Alcohol Depend 166: 32–38.
Washington State Marijuana Impact Report (2016). Available at http://www.riag.ri.gov/documents/NWHIDTAMarijuanaImpactReportVolume1.pdf Accessed 2 July 2017.
The Washington Times (2016). Marijuana sales in Washington state top $1 billion: Report. Available at: http://www.washingtontimes.com/news/2016/jul/8/marijuana-sales-washington-top-1-billion-report/?_sm_au_=iVVwFVZDW6Q7kD46 Accessed 30 June 2017.
Watson TM, Mann RE (2016). International approaches to driving under the influence of cannabis: a review of evidence on impact. Drug Alcohol Depend 169: 148–155.
Weiss SRB, Blanco C, Wargo EM (2017). Clarifying the link between cannabis use and risk for psychosis. Acta Psychiatr Scand 136: 3–4.
Wen H, Hockenberry JM, Cummings JR (2015). The effect of medical marijuana laws on adolescent and adult use of marijuana, alcohol, and other substances. J Health Econ 42: 64–80.
Wettlaufer A, Florica RO, Asbridge M, Beirness D, Brubacher J, Callaghan R et al (2017). Estimating the harms and costs of cannabis-attributable collisions in the Canadian provinces. Drug Alcohol Depend 173: 185–190.
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV et al (2015). Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313: 2456–2473.
Wilkinson ST, Stefanovics E, Rosenheck RA (2015). Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry 76: 1174–1180.
Williams AR, Olfson M, Kim JH, Martins SS, Kleber HD (2016). Older, less regulated medical marijuana programs have much greater enrollment rates than newer 'medicalized' programs. Health Aff (Millwood) 35: 480–488.
Williams AR, Santaella-Tenorio J, Mauro CM, Levin FR, Martins SS (2017). Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. doi: 10.1111/add.13904.
World Health Organization (1992) International Statistical Classification of Diseases and Related Health Problems, 10th revision. ed. World Health Organization: Geneva.
Wu CS, Jew CP, Lu HC (2011). Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol 6: 459–480.
Wu LT, Zhu H, Mannelli P, Swartz MS (2017). Prevalence and correlates of treatment utilization among adults with cannabis use disorder in the United States. Drug Alcohol Depend 177: 153–162.
Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, Harding IH et al (2012). Effect of long-term cannabis use on axonal fibre connectivity. Brain 135 (Pt 7): 2245–2255.
Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G (2002). Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 325: 1199.
Zhu H, Wu LT (2016). Trends and correlates of cannabis-involved emergency department visits: 2004 to 2011. J Addict Med 10: 429–436.
Acknowledgements
Lili Sar-Graycar and Brittney Davis are thanked for their editorial assistance. This work was supported by a grant from the National Institute on Drug Abuse (R01DA034244) and the New York State Psychiatric Institute.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hasin, D. US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacol. 43, 195–212 (2018). https://doi.org/10.1038/npp.2017.198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2017.198
This article is cited by
-
“Everything is kind of the same except my mind is with me”: exploring cannabis substitution in a sample of adults in early recovery from an opioid or stimulant addiction
Harm Reduction Journal (2024)
-
State cannabis laws and cannabis positivity among fatally injured drivers
Injury Epidemiology (2024)
-
Association of semaglutide with reduced incidence and relapse of cannabis use disorder in real-world populations: a retrospective cohort study
Molecular Psychiatry (2024)
-
Abnormal developmental of structural covariance networks in young adults with heavy cannabis use: a 3-year follow-up study
Translational Psychiatry (2024)
-
A Latent Class Analysis of Age at Substance Use Initiation in Young Adults and its Association with Mental Health
International Journal of Mental Health and Addiction (2024)