Abstract
Increasing data indicate that inflammation and alterations in glutamate neurotransmission are two novel pathways to pathophysiology in mood disorders. The primary goal of this review is to illustrate how these two pathways may converge at the level of the glia to contribute to neuropsychiatric disease. We propose that a combination of failed clearance and exaggerated release of glutamate by glial cells during immune activation leads to glutamate increases and promotes aberrant extrasynaptic signaling through ionotropic and metabotropic glutamate receptors, ultimately resulting in synaptic dysfunction and loss. Furthermore, glutamate diffusion outside the synapse can lead to the loss of synaptic fidelity and specificity of neurotransmission, contributing to circuit dysfunction and behavioral pathology. This review examines the fundamental role of glia in the regulation of glutamate, followed by a description of the impact of inflammation on glial glutamate regulation at the cellular, molecular, and metabolic level. In addition, the role of these effects of inflammation on glia and glutamate in mood disorders will be discussed along with their translational implications.
Similar content being viewed by others
INTRODUCTION
Two emerging theories regarding the development of mood disorders involve excessive activation of inflammatory pathways and alterations in glutamate metabolism (Dantzer et al, 2008; Haroon et al, 2012, 2016; Sanacora and Banasr, 2013; Sanacora et al, 2012). Recent evidence indicates that these two pathways converge at the level of the glia to result in behavioral alterations in patients with mood disorders. Indeed, data indicate that inflammatory mediators might regulate extracellular glutamate concentrations by exerting profound effects on the functioning of glial cells including astrocytes, oligodendrocytes, and microglia that regulate glutamate levels under both physiological and pathological conditions.
In this review, we propose the hypothesis that the effects of inflammation on glia initially lead to an increased release and ‘spillover’ of glutamate into the extrasynaptic space by decreasing the capacity of glial transporters to buffer and clear glutamate (McCullumsmith and Sanacora, 2015; Figure 1). This glutamate spillover in combination with glutamate released by activated or primed glial and immune cells can activate extrasynaptic N-methyl-D-aspartate (NMDA) receptors and lead to atrophy and regression of dendritic spines and processes and loss of synaptic integrity, ultimately resulting in neuronal loss (Hardingham and Bading, 2010; Malarkey and Parpura, 2008). Moreover, increases in extrasynaptic glutamate can also stimulate presynaptic metabotropic glutamate receptors (mGluR2/3) leading to reductions in synaptic glutamate transmission (Duman, 2014; McEwen et al, 2016). In addition, alterations in the ratio between synaptic and extrasynaptic activation of ionotropic receptors by glutamate can result in the loss of synaptic fidelity and specificity of neurotransmission, contributing to circuit dysfunction (Allam et al, 2015; Allam et al, 2012; Figure 1). This process might however be reversed if treatments or spontaneous recovery processes engage astroglial repair and plasticity promoting mechanisms. Taken together, these effects of inflammation on glial cells can have a profound effect on glutamate neurotransmission, which may ultimately serve as a fundamental mechanism by which inflammation influences behavior in patients with mood disorders.
Impact of inflammation on glutamate neurotransmission and synaptic integrity. Left panel depicts the glutamatergic synapse at an early stage of inflammation. High levels of inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin (IL)-1β released by activated inflammatory cells including microglia, astroglial, and macrophages lead to increases in the concentration of synaptic glutamate (GLU), toxic kynurenine molecules with glutamate-like effects such as quinolinic acid (QUIN), and reactive oxygen (ROS)/nitrogen (RNS) species leading to oxidative stress. Effects of inflammatory molecules upon astrocytic cell morphology leads to decreased ability to ‘cradle’, sequester, and contain glutamate within the synapse resulting in a spillover of the glutamate into the extrasynaptic space. Concurrent decreases in the number and functioning of excitatory amino-acid transporters (EAAT) 2-induced by inflammation further limits the ability of astrocytes to buffer and clear the spillover glutamate. Additional release of glutamate by increased xC-activity alone or in combination with reverse effluxive release via EAATs by immune-activated glial cells further adds to the concentrations of extrasynaptic glutamate available to diffuse and bind to extrasynaptic binding sites. Increases in synaptic glutamate resulting from early inflammatory changes are shown as leading to the overactivation of intrasynaptic ionotropic receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate receptors (NMDA) potentially contributing to excitotoxicity. The spillover glutamate is also shown as binding to the extrasynaptic NMDA receptors leading to suppression of neurotrophic support from factors such as brain-derived neurotrophic factor (BDNF). Some of the excess glutamate is also seen binding to presynaptic metabotropic glutamate receptors (mGluR2/3), which provides negative feedback to block further glutamate release as shown in the right panel. Right panel depicts the glutamate synapse at a late stage of chronic inflammation. The progression of glutamatergic dysfunction has resulted in an overall loss of glutamate neurotransmission. Sustained stimulation of presynaptic mGluR2/3 systems by glutamate diffusing through extrasynaptic space leads to inhibition of excitatory neurotransmission and increased trapping of glutamate in the vesicles within the presynaptic membrane. The toxic effect of overstimulation of intrasynaptic AMPA during early immune activation has led to a downregulation, desensitization, and atrophic loss of intrasynaptic AMPA receptors. Decreasing intrasynaptic glutamatergic activity also leads to the loss of intrasynaptic NMDA signaling even while the extrasynaptic NMDA signaling continues to remain high. This altered ratio of intrasynaptic to extrasynaptic signaling leads to atrophy and regional loss of neurons seen in patients with mood disorders as seen in the shrinking postsynaptic neuron. Finally, the persistently increased extrasynaptic glutamate, increased ROS/RNS, and QUIN also contribute to astrocytic and oligodendrocyte toxicity leading to atrophic changes in these cells as well.
This review will examine the impact of inflammation on glutamate neurotransmission by first describing the role of glia in the regulation of glutamate, followed by a description of the impact of inflammation on glial glutamate regulation at the cellular, molecular, and metabolic level. In addition, a working model will be presented and the impact of inflammation on glial glutamate regulation will be discussed in the context of its translational relevance.
CELLULAR BASIS OF INTERACTIONS BETWEEN THE IMMUNE SYSTEM AND GLUTAMATE NEUROTRANSMISSION
To fully understand the intersection between glutamate and inflammation as they relate to mood disorders, it is essential to first describe the glial elements that serve as the point of convergence of these interactions.
ASTROCYTES
Historically, astrocytes were viewed as merely supporting cells providing structural support to the neuronal network much like the connective tissue in the periphery. However, it is now well established that astrocytes carry out a myriad of functions including providing neurotrophic support, maintaining synaptic homeostasis, regulating synaptic pruning, participating in neuron–glia signaling, coordinating neurometabolic coupling, mitigating oxidative stress, and the most relevant to the purpose of this review, modulating glutamate metabolism (Kimelberg and Nedergaard, 2010; Verkhratsky et al, 2015a). A brief overview of these functions is presented here, although the reader is referred to other, more comprehensive texts for a more detailed description of the various functions of astrocytes (Kettenmann and Ransom, 2012; Nedergaard et al, 2002; Verkharatsky and Butt, 2013; Verkhratsky et al, 2015a; Verkhratsky et al, 2014; Volterra and Meldolesi, 2005).
In stark contrast to the myeloid/bone marrow origins of microglia, astrocytes (and oligodendrocytes) are derived from neural stem cells belonging to the embryonic, ectodermal plate, and neural crest. As part of the tripartite synapse that includes the synaptic regions of the pre- and postsynaptic neurons and associated astrocytic processes, the primary role of astrocytes is to maintain synaptic integrity (Haydon and Carmignoto, 2006; Nedergaard et al, 2002). Indeed, although astrocytic dysfunction and resulting synaptic loss might define the pathology of several neuropsychiatric and neurodegenerative disorders (such as depression and dementia), aberrant synapse formation has also been implicated in a number of neurodevelopmental disorders including schizophrenia, autism, and Fragile-X syndrome (Duman et al, 2016; Mateo et al, 2011).
Astrocytes are multi-branched stellate cells, whose morphology and functions tend to change with varying degrees of specialization and differentiation, often in response to physiological and pathological demands including immune activation. At least three morphological subtypes of astrocytes are recognized in the brain including protoplasmic, fibrous, and radial astrocytes (Liddelow and Barres, 2015; Sofroniew and Vinters, 2010).
Protoplasmic astrocytes are found throughout all gray matter and exhibit a branching morphology with several stem-like processes that give rise to branches and further divide into progressively finer processes, which at one end encase blood vessels through their endfeet and at the other end ensheath several thousand synapses forming ‘astrocytic cradles’ (Bushong et al, 2002; Sofroniew and Vinters, 2010). The ‘astrocytic cradles’ that encircle the synaptic region are densely enriched with glutamate transporters and have a primary role in glutamate clearance under physiological conditions (Khakh and Sofroniew, 2015; Liddelow and Barres, 2015; Sofroniew and Vinters, 2010). The glutamate is cleared primarily through highly efficient excitatory amino-acid transporters (EAAT1–2) located on astrocytes in the ‘cradles’ (Nedergaard et al, 2002; Verkhratsky et al, 2015a). Once taken up into the astrocytes, glutamate is rapidly converted into the ‘inert’ intermediate glutamine, which is then released and absorbed by neurons, where it is converted back into glutamate and packaged into synaptic vesicles through vesicular glutamate transporters (VGLUT1–3), completing the glutamate/glutamine metabolic cycle (Danbolt, 2001; Zhou and Danbolt, 2014). The recycled glutamate serves as the principle source of synaptically released glutamate for neurotransmission. In addition to providing a mechanism of efficiently recycling extracellular glutamate, the ‘astrocytic cradle’ creates an anatomical and functional barrier that blocks the ‘spillover’ of glutamate into the extrasynaptic space (Dorsett et al, 2016; McCullumsmith and Sanacora, 2015). Although glutamate binds to EAATs with high affinity, not all the bound glutamate is immediately transported across the plasma membrane of astrocytes (Dorsett et al, 2016). EAAT-bound glutamate may become ‘unbound’, released, and rebound to neighboring glutamate transporters, thus bouncing from one transporter to the other until it is eventually transported across the plasma membrane (Tzingounis and Wadiche, 2007). This process of the temporary holding of glutamate before transport is referred to as ‘buffering’ and it helps limit its spillover outside the cradle (Dorsett et al, 2016). Finally, through their podocytic endfeet, astrocytes come in close proximity to blood vessels and vascular pericytes, leaving an empty space—a ‘no man’s land’ of extracellular space that forms an integral part of the blood–brain barrier (BBB) and brain lymphatic systems (Iliff and Nedergaard, 2013; Louveau et al, 2015). In summary, functions of the protoplasmic astrocytes and their astrocytic cradles are critical in maintaining amino-acid metabolic homeostasis, preventing excessive glutamate spillover and preserving the BBB.
Fibrous astrocytes are found throughout white matter regions and exhibit many elongated fiber-like processes, which encircle the nodes of Ranvier (Barres, 2008; Sofroniew and Vinters, 2010). These fibrous astrocytic processes protect and nurture the unmyelinated nodal regions as opposed to the oligodendrocytes that protect and nurture the myelin sheath and myelin cells (Khakh and Sofroniew, 2015; Sofroniew and Vinters, 2010). This unique spatial arrangement of astrocytes and oligodendrocytes enables them to interact and promote axonal and dendritic plasticity. In addition to performing all functions of protoplasmic astrocytes that are the most common form of astrocytes, the fibrous astrocytes also have a prominent role in repair of damaged neurons and lead to scar formation (Sofroniew and Vinters, 2010). A final type of astrocyte is the ‘radial’ astrocyte, which develops from neural stem cells during early embryogenesis to assist in neuronal migration and in the development of neural plate. The radial astrocyte is known to persist in the adult retina and cerebellum as Mυller and Berger cells respectively (Khakh and Sofroniew, 2015; Liddelow and Barres, 2015).
Immune Regulation
Astrocyte function is modulated by a variety of immune processes. Astrocytes express receptors for multiple immune-derived molecules including cytokines, chemokines, complement, and acute-phase proteins, and respond to immune signals generated by microglia or peripheral macrophages recruited to into the brain during innate immune activation. In addition, astrocytes express receptors for adipokines such as the leptin receptors (LepR), which can be upregulated in obesity and inflammation and may have a role in appetite regulation (Hsuchou et al, 2009; Pan et al, 2012). By regulating the BBB, astrocytes also act as ‘gate keepers’ to control peripheral immune cell trafficking to the brain (Abbott et al, 2006).
Acute activation
During pathologic conditions associated with large-scale cell death such as ischemia, oxidative stress, or neural trauma, astrocytes themselves can become inflammatory cells and demonstrate phagocytic activity (Fontana et al, 1984; Soos et al, 1998). Transformation of astrocytes into phagocytic and antigen-presenting cells (as evidenced by local increases of surface markers of inflammatory activation including major histocompatibility complex (MHC) class II receptors, toll-like receptors (TLRs), and intracellular immune receptor systems including the NOD-like protein 3 (NLRP3) or Rig-1-protein complex of the inflammasome) is triggered by local increases in concentrations of inflammatory cytokines such as interferon (IFN)-γ (de Rivero Vaccari et al, 2014, 2016b; Farina et al, 2005, 2007). The inflammasome is an intracellular complex that senses danger-associated molecular patterns (DAMPs) such as ATP that are released during cellular stress. DAMP signaling then leads to the activation of caspase 1, which in turn cleaves pro-IL-1 into active IL-1β (Walsh et al, 2014a). Upon activation by IL-1β, astrocytes can release large amounts of innate immune inflammatory mediators including several complement cascade proteins; cytokines including tumor necrosis factor (TNF), IL-1β, IL-6; and chemokines with CC (cysteine–cysteine) and CXC (cysteine–other amino acids–cysteine) motifs including CCL2, CXCL1, CXCL10, and CXCL12 (Bezzi et al, 2001; Fischer et al, 2014; Johnstone et al, 1999; Marz et al, 1999; Olmos and Llado, 2014; Santello and Volterra, 2012; Takahashi et al, 2003a; Torres-Platas et al, 2014b; Xie et al, 2003). This state is also associated with impaired glutamate clearance and oxidative stress, both of which contribute to excitotoxicity (Matute et al, 2006; Tilleux and Hermans, 2007; Zou and Crews, 2005).
Chronic activation (reactive astrocytosis)
Astrocytes respond to chronic CNS injuries and diseases by differentiating and proliferating into hypertrophic and/or migrating cell types—a term referred to as reactive astrocytosis (Khakh and Sofroniew, 2015; Liddelow and Barres, 2015; Sofroniew and Vinters, 2010). Reactive astrocytosis may have both reparative functions that preserve synaptic homeostasis (eg, increasing the secretion of neurotrophic factors such as nerve growth factor, brain-derived neurotrophic factor [BDNF], and activity-dependent neurotrophic growth factor, and increasing glutamate clearance through cradling and reuptake mechanisms) or toxic functions resulting in neuronal loss (Friedman et al, 1990; Liberto et al, 2004; Marz et al, 1999; Spranger et al, 1990). Accordingly, reactive astrocytes can be classified into A1 and A2 astrocytes (Karpuk et al, 2012).
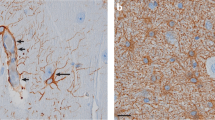
A2 reactive astrocytes were demonstrated to promote reparative healing after following ischemic injury induced by an experimental stroke lesion in a beneficial manner (Zamanian et al, 2012). Following ischemic injury, A2 reactive astrocytosis was associated with a rapid but quickly attenuated induction of the expression of two genetic markers—Lcn2 and Serpina3n—both of which were co-localized with the glutamate transporter gene (Zamanian et al, 2012). On the other hand, A1 phenotype was induced by inflammatory stimuli including experimental administration of endotoxin and was more pathological in nature (Zamanian et al, 2012). A1 astrocyte pathology falls into two general categories: loss-of-function type, in which astrocytes fail to perform normal functions (decrease glutamate clearance and/or decrease synaptogenesis) or gain-of-function type in which astrocytes exhibit new, potentially deleterious, functions (increased synaptic destruction) not seen in resting astrocytes (Khakh and Sofroniew, 2015; Sofroniew and Vinters, 2010). The neurotoxic effects of A1 reactive astrocytes were mediated by the upregulation of complement cascade components, glutamate-mediated excitotoxicity, and induction of oxidative stress (Zamanian et al, 2012). Of note, increases in genetic markers of reactive astrocytosis, namely, glial fibrillary acidic protein (GFAP) and aldehyde dehydrogenase 1 family member L1 (ALDH1L1), have been reported in multiple brain regions of patients with bipolar disorder (Barley et al, 2009).
Senescence-associated secretory phenotype
Astrocytes undergo phenotypic conversion during aging and can develop into the senescence-associated secretory phenotype (Salminen et al, 2011). This senescent phenotype exhibits exaggerated reactivity to immune activation and is associated with reduced glutamate clearance as well as the production of inflammatory cytokines (Haroon et al, 2015; Norden and Godbout, 2013).
Synaptogenesis, Gliotransmission, and Circuit Integration
Synaptogenesis
Synapses are formed by synapse-forming proteins secreted by astrocytes such as glypicans (Liddelow and Barres, 2015). Glypicans amplify the intensity of neurotransmission by increasing the expression and activity of postsynaptic AMPA receptors. Thus, the voltage of the postsynaptic current is amplified to a level sufficient enough to open the magnesium-gated NMDA receptors resulting in the establishment of synaptic connections (Liddelow and Barres, 2015). Once established, the synapse is maintained through a coordinated program of immune-glutamate signaling involving mGluRs, chemokines, and TNF. Some of the glutamate released during neurotransmission diffuses and binds to surface mGluRs on astrocytes and trigger release of the chemokine CXCL12/stromal cell-derived factor (CXCL12/SDF1), which in turn signals the microglia to release small, physiologic (non-inflammatory) quantities of TNF (Heinisch and Kirby, 2010; Santello and Volterra, 2012). The released TNF binds to TNF receptors on astrocytes and enables regulated buffering and clearance of glutamate, thereby maintaining controlled neuronal excitation. This process of immune-to-glutamate signaling ensures that EAAT uptake is not an all or none phenomenon, but a more nuanced, synapse-strengthening process. Recently, direct evidence implicating CXCL12/SDF1 in preclinical models of anxiety and other neuropsychiatric disorders have been published (Guyon, 2014; Yang et al, 2016). Astrocytes have recently been implicated to have a role in synaptic elimination during brain development in a manner similar to the role played by the microglia in developmental pruning (Chung et al, 2013).
Postsynaptic density proteins (PSD95) are scaffolding proteins that cluster, hold, and connect AMPA and NMDA receptor proteins to their downstream signaling molecules such as stargazin and D-serine (Ma et al, 2014; Sheng and Hoogenraad, 2007). The clustering and location of ionotropic receptors within the postsynaptic membrane varies across contexts and time points, and regulates the balance between activation and desensitization of AMPA and NMDA receptors, and thus has the potential to decrease neuroplasticity and increase signaling disarray (Allam et al, 2015; Allam et al, 2012; Ma et al, 2014). PSD95 function is controlled by immune activity in the glial cells such as astrocytes and microglial cells (Torres-Platas et al, 2014b; Torres-Platas et al, 2011) during physiological (normal development) and pathological contexts (Estes and McAllister, 2015). For example, prenatal immune activation using poly(I:C), a preclinical model of prenatal viral exposure implicated in schizophrenia and autism spectrum disorders resulted in pronounced decreases of PSD95 in hippocampal regions associated with prominent behavioral changes (Giovanoli et al, 2016). Rapid antidepressant effect of ketamine has been linked with increases in PSD95 (Duman and Voleti, 2012). Postmortem studies using brain specimens from depressed suicide victims have revealed epigenetic modifications (methylation abnormalities) in the promoter region of BEGAIN, a gene associated with PSD95 expression (Nagy et al, 2015). In addition, PSD95 engulfment by microglia can lead to excessive complement activation and pruning (Estes and McAllister, 2015). Although specific information is still lacking, it would be tempting to speculate that the movement and clustering of AMPA and NMDA proteins and their binding to stargazin or nitric oxide synthase within the PSD95 complex might be altered during inflammatory activation in mood disorders.
Gliotransmission
Signaling between glial cells also known as gliotransmission is known to have a role in maintaining synaptic tone, guiding neuronal development, and engage in constructive pruning (Kielian and Esen, 2004; Petrelli and Bezzi, 2016). Glial signaling is facilitated by direct transfer of neurotransmitters (such as glutamate) and ions (such as Ca+ and Na+) from one glial cell to another through gap junctions, which are essentially intracellular channels encircled by six transmembrane proteins known as connexins (Cx; Kielian and Esen, 2004). Thus, connections between distal process of neighboring astrocytes and oligodendrocytes and even microglia signal each other to convey useful information about the microenvironment. Large numbers of astrocytes connect with one another through such gap junctions to form connected astroglial syncytia capable of transmitting signaling information transynaptically through large regions of the brain, thus placing astrocytes as hubs or intense brain activity (Volterra and Meldolesi, 2005). Neuronal presynaptic glutamate release triggers waves of intracellular Ca+ and Na+ oscillatory activity and inwardly rectifying currents in neighboring astrocytes, which is then propagated throughout the syncytium leading to glutamate release and reuptake changes in distant sites (Innocenti et al, 2000; Parri et al, 2001). Of note, immune signals are also propagated through gap junction communication between microglia and astrocytes (Kielian et al, 2004; Kielian and Esen, 2004). Immune mediators such as TNF modulate functioning and vitality of gap junctions (Santello and Volterra, 2012; Takeuchi and Suzumura, 2014). The role of the immune-related gap junction pathology in propagating neural activity through rapid transfer of glutamate has been blamed for the rapid dissemination of seizure discharges and the resulting kindling effects seen seizure disorder (Iori et al, 2016; Vezzani and Viviani, 2015; Viviani and Boraso, 2011). Mood disorders are known to be associated with Cx pathology and this remains an ongoing area of investigation (Miguel-Hidalgo et al, 2014).
Circuit integration
Recent work suggests the current concept of brain networks, as intelligent contacts between several neurons should be expanded to include communications occurring between neurons and glial cells (Rossi, 2015; Santello et al, 2011; Volterra and Meldolesi, 2005). Astrocytes are best viewed as local ‘hubs’ that can receive and integrate information from thousands of synapses, other glial cells and blood vessels (Bushong et al, 2002; Rossi, 2015; Santello et al, 2011; Volterra and Meldolesi, 2005). In contrast to neural circuits, astrocytic networks establish connections among unrelated and functionally segregated circuits (Bushong et al, 2002; Sofroniew and Vinters, 2010; Volterra and Meldolesi, 2005). Furthermore, astrocytes accomplish this integrative function using a combination of gliotransmitters such as glutamate and physiological concentrations of cytokines such as TNF as described above.
There is increasing evidence suggesting that the integrated function of these glial networks can become pathological in disease states. For instance, glutamate (along with dopamine) has been implicated in regulation of physiological reward-seeking behaviors and pathological anhedonic states (Bechtholt-Gompf et al, 2010; Whitton et al, 2015). A circuit level competition between midbrain dopaminergic circuits (mediating reward experience) and medial prefrontal cortex-based glutamatergic circuits (mediating reward suppression) for the control of a third circuit (ventral striatum) to guide hedonic responses and stimulus salience in physiology and anhedonic responses in depression has been proposed (Ferenczi et al, 2016). Intriguingly, disruption of astrocytic glutamate uptake/clearance using EAAT2 inhibitor dihydrokainic acid in rodents was associated with reduced sensitivity to reward in intracranial self-stimulation paradigms implicating the role of glutamate increases in disrupting reward pathways (Bechtholt-Gompf et al, 2010). Further evidence suggesting that abnormal function of glia networks can contribute to mood disorder pathophysiology comes from a study demonstrating that dysfunction of the orbitofrontal cortex in MDD and alcohol abuse is associated with a reduction in the levels of gap junction protein Cx43, implicating gap junction in the pathophysiology of circuit dysfunction. Thus, using a dimensional perspective based on the RDOC initiative (Insel et al, 2010), it is possible to hypothesize a balance between glutamatergic and dopaminergic systems representing opposing polarities of salience, ie, ‘negatively valent/reward suppressive’ vs ‘positively valent/reward experience’ states. Given the impact of inflammation on both of these polarities, it is likely that inflammation-induced circuit pathologies will be an important area of future investigation (Felger et al, 2016; Haroon et al, 2016).
Astrocytic Dysfunction and Mood Disorders
A robust literature exists supporting the association between astrocytic dysfunction and mood disorders in general, and has been extensively reviewed elsewhere (Ongur et al, 2014; Popoli et al, 2012; Rajkowska and Miguel-Hidalgo, 2007; Rajkowska and Stockmeier, 2013; Sanacora and Banasr, 2013; Verkhratsky et al, 2014). Major highlights of this literature are provided in Box 1.
OLIGODENDROCYTES
Oligodendrocytes form an integral part of the brain white matter and have critical roles in maintaining the convergence of excitatory neurotransmission, glutamate homeostasis, and immune dysregulation at the level of the white matter tissue. Oligodendrocytes produce myelin that provides critical insulation to enable faster conduction speeds compared with unmyelinated axons of the same diameter (Bartzokis, 2004; Edgar and Sibille, 2012). Oligodendrocytes have small round cell bodies surrounded by four to six branching processes that are the myelinating regions and can myelinate (or remyelinate following injury) up to 60 axons (Edgar and Sibille, 2012). Based on their location, two distinct types of oligodendrocytes have been described as follows: (1) perineural oligodendrocytes found primarily in the gray matter in close proximity to neuronal cell bodies; and (2) interfascicular oligodendrocytes that are found in the white matter, which ensheath axonal fibers (Edgar and Sibille, 2012). Myelin is synthesized by myelin-related genes in the oligodendrocytes using a combination of lipids (70%) and proteins (30%) including myelin basic protein throughout most of adult life (Chen et al, 2013). The declining trajectory of myelination has been correlated with decreases in cerebral function as happens during aging (Bartzokis, 2004).
Oligodendrocytes Promote Excitatory Neurotransmission
Oligodendrocytes have an impact on excitatory neurotransmission and promote neural and circuit plasticity in concert with astrocytes (Gautier et al, 2015; Jebelli et al, 2015). Myelination of axons is dependent on neuronal activity and continues well into older adult life, underlining a role for oligodendrocytes in homeostatic (aging) and pathological contexts (demyelination and neural injury; Gautier et al, 2015; Jebelli et al, 2015). Oligodendrocytes control myelinated regions of the axon, whereas nodal astrocytes regulate the intervening nodes of Ranvier, which are spaces of bare axon enriched with ion channels, amino-acid transporters and their receptors (Edgar and Sibille, 2012). Together the oligodendrocytes and nodal astrocytes regulate and control the speed of ‘saltatory conduction’, thus maintaining optimal speed of information transfer across neurons and their networks. Activity-dependent axonal plasticity involves communication between myelinating oligodendrocytes and nodal astrocytes using glia–glia signaling probably through gap junctions (Ishibashi et al, 2006). These activity-dependent increases in myelination and plasticity seen among older individuals and points to the utility of neural entraining and stimulation techniques to reverse age-associated declines (Merzenich et al, 2014).
Oligodendrocytes and Glutamate
Similar to neurons, oligodendrocytes are highly vulnerable to glutamate toxicity. Glutamate toxicity to oligodendrocytes may be precipitated by oxidative stress or direct ionotropic receptor-mediated excitotoxicity (Oka et al, 1993; Takahashi et al, 2003b). Oligodendrocyte cell toxicity might be mediated by the overstimulation of AMPA and kainate receptors located on the oligodendrocyte cell body, whereas impaired myelin synthesis might result from toxic activation of NMDA receptors densely represented in the branching processes (Matute et al, 2006; Verkharatsky and Butt, 2013). Although cell death from glutamate overactivation of AMPA/kainite receptors is of interest in the study of demyelinating disorders, toxicity from oligodendrocytic NMDA receptors might be of interest to psychiatry, as these receptors are located on the oligodendrocyte branches that are active myelin-synthesizing regions (Matute, 2006). Similar to astrocytes, increases in inflammatory cytokines such as TNF and IL-1β are known to impair glutamate buffering and clearance by oligodendrocyte EAATs, and trigger glutamate toxicity (Takahashi et al, 2003a; Takahashi et al, 2003b). Indeed, inhibition of the expression and functioning of glutamate transporters such as EAATs and xC-transporters in axonal tracts is sufficient to induce oligodendrocyte loss and demyelination, which undermines brain connectivity (Domercq et al, 2007; Evonuk et al, 2015).
Oligodendrocytes and Mood and Other Disorders
Varying degrees of myelin loss and decreasing function and/or numbers of oligodendrocytes have been reported among patients with mood disorders and schizophrenia (Edgar and Sibille, 2012; Rajkowska et al, 2015; Tavares et al, 2002). Oligodendrocyte cells contain substantial quantities of iron, which make them highly vulnerable to oxidative stress through the generation of reactive oxygen species. Transferrin—an iron binding, mobilization, and detoxification protein—was underexpressed in the oligodendrocyte cells in the internal capsule of patients with bipolar disorder (Barley et al, 2009). Of note, transferrin is also an acute-phase protein, which is depleted during immune activation (Ritchie et al, 1999). Oligodendrocyte loss accounts for a high proportion of the cell loss in the amygdala in major depression (Hamidi et al, 2004).
NG2+ Glial Cells
NG2+ cells are a specific subtype of oligodendrocyte precursor cells that continue to exist and function in the adult brain (Woodbury-Farina, 2014). NG2+ glia cells connect with axons using primitive glutamatergic synapses and probably direct the formation of both glutamatergic and GABAergic synapses during the brain development before myelination (Dimou and Gallo, 2015). Exposure to preclinical stress paradigms led to decreases in number and density of NG2+ cells among lab animals (Birey et al, 2015) and loss of NG2+ was associated with decreased astrocytic EAATs activity and glutamate increases in prefrontal and hippocampal regions (Banasr et al, 2010; Birey et al, 2015). The depression-like symptoms associated with NG2+ loss responded to antidepressant medications and electroconvulsive treatments, both of which are known to alter glutamatergic neurotransmission in addition to other neurotransmitters (Banasr and Duman, 2008; Banasr et al, 2007; Sanacora and Banasr, 2013). Under conditions of immune activation, NG2+ cells are known to transform into both astrocytes and oligodendrocytes (Dimou and Gallo, 2015). The role of NG2+ cells in maintaining neural plasticity and promoting behavioral resilience warrants further study.
MICROGLIA
Microglia are the resident immune cells of the brain. Similar to macrophages in the peripheral blood, microglia arise from primitive myeloid cells in the embryonic mesoderm of the yolk sac, but migrate into the neural plate during a narrow time window before vascularization or definitive hematopoiesis (Ginhoux et al, 2010). However, unlike macrophages, microglia reside and function within the brain parenchyma outside the limits of peripheral circulation. Once established in the brain parenchyma, microglial cell lines are sustained by the proliferation of resident microglial progenitor cells. Microglial activity and its morphology is highly context-sensitive and depends on the spatial and temporal distribution of the physiological or pathogenic influences (Fenn et al, 2014; Figuera-Losada et al, 2014; Kettenmann and Ransom, 2012; Reus et al, 2015; Torres-Platas et al, 2014a; Torres-Platas et al, 2014b). However, defining and staging microglial activation states have been complicated by the lack of unbiased insights into the contextual factors driving authentic microglial reaction states (Ransohoff, 2016). The contextual challenges may include a varying combination of neurological and immunological stimuli. In addition to their macrophage-like immune-surveillance functions, microglia also exhibit glial/trophic functions and actively respond to the changes in neural activity (Gras et al, 2012). Consequently, for the purpose of this review, it becomes necessary to define the stages of microglial activation despite the arbitrariness surrounding this discussion. Thus, the staging of microglial activation used in this discussion should be viewed as an oversimplification to facilitate the data interpretation, whereas a more comprehensive staging encompassing immunological and neurotrophic functions is awaited (Figure 1).
Surveillance State
Microglia are mobile cells, which possess motile ramified processes enriched with immune receptors that continually protrude and withdraw to scan their microenvironment for immune signals (Eggen et al, 2013; Nimmerjahn et al, 2005). Microglia in this ‘surveillance state’ can move around and scan the entirety of the brain parenchyma within a matter of hours and hence the name ‘resting’ state has been discarded.
Classic Activation State
Upon activation, microglia exhibit varying morphologies and activation patterns ranging from the classical pro-inflammatory and neurotoxic to the alternative anti-inflammatory and neuroprotective profiles (Kettenmann et al, 2011; Pocock and Kettenmann, 2007; Ransohoff, 2016). Inflammatory or ‘classical activation’ results from binding of microbial pathogen-associated molecular patterns to microglial TLRs and activation of inflammasome NLRP3 by DAMPs (eg, binding of ATP to the purinergic receptor P2X7) released by stressed, damaged or dead cells. The ‘classical activation’ state is associated with high levels of inflammatory gene expression and secretion of inflammatory cytokines such as IL-1β, TNF, IL-6, IL-12, and IFN-γ as well as chemokines such as CCL2 that attract peripheral monocytes into the brain and are increased in perivascular regions of postmortem brain specimens from depressed suicide victims (D’Mello et al, 2009; Torres-Platas et al, 2014b; Wohleb et al, 2014). Morphologically, these cells adopt a deramified (reactive) or amoeboid appearance (with one or less process) and move toward regions of injury (Torres-Platas et al, 2014a). Upon activation, microglial cells also express phagocytic profile characterized by the surface expression of antigen-presenting markers such as MHC class II. Of note, increased or de novo surface expression of glutamate receptors and transporters is seen exclusively during this phase of activation. Microglia and trafficking macrophages can be release large quantities of glutamate in the extrasynaptic space along with glutamate-like toxic kynurenines (KYNs) such as quinolinic acid (QUIN; Table 1).
Alternative Activation and Acquired Deactivation States
A putative ‘alternative activation’ state has also been proposed as a predominantly anti-inflammatory phenotype seen in response to IL-4 or IL-13 (Wei and Jonakait, 1999). However, these microglia are still phagocytic but limited to clearing damaged tissue, misfolded proteins (such as β-amyloid), and other debris (Graeber and Streit, 2010; Miller and Streit, 2007; Streit and Xue, 2013), and do not possess antigen-presenting characteristics such as the surface expression of activation markers (MHC class II and cluster of differentiation (CD)40 (Town et al, 2005). In addition to producing anti-inflammatory cytokines such as IL-4, IL-10, and IL-13, these cells are also capable of producing growth factors such as transforming growth factor (TGF)-β and can serve a neuroprotective role (Town et al, 2005). A subtype known as ‘acquired deactivation’ state during which the cells show combined features of both ‘surveillance’ and muted but persisting activation states characterized by phagocytic and immune responses has also been described (Town et al, 2005). Taken together, the above data indicate that microglial cells form the backbone of innate immunity signaling and are often seen in varying levels of activation and rest. As will be seen later, these cells also engage in both glutamate clearance and release but only during activated states.
Microglia priming and chronic activation
Recent evidence suggests that activated microglia can reside in an in-between state of partial activation known as priming characterized both by increased responsivity to immune stimulation and persistent, chronic elevations of baseline cytokine secretion during unstimulated conditions (Dilger and Johnson, 2008; Godbout and Johnson, 2009; Godbout et al, 2008). This phenomenon of priming requires both a priming stimulus (such as chronic activation by IFN-γ) and a secondary but acute triggering stimulus such as TLR binding by bacterial antitoxins (Eggen et al, 2013; Fenn et al, 2014). The priming stimulus appears to cause a phenotypic shift towards a more sensitized state characterized by the increased expression of MHC class II and increased glutamate release. The primed cell will then mount a more exaggerated and rapid response to the triggering stimulus than a non-primed cell. Several conditions of priming exist. For instance, aged rodents demonstrate increased expression of mRNA and protein markers of inflammation following LPS challenge (Kumar et al, 2013; Norden and Godbout, 2013; Norden et al, 2015a, 2015b). In addition to aging, similar priming effects have been reported in microglia from patients with neuropsychiatric disorders including traumatic brain injury (Fenn et al, 2014) and neurodegeneration (Norden et al, 2015a). Of note, primed microglia were less responsive to the effects of glutamate reuptake enhancing agent riluzole compared with unprimed microglia from younger individuals (Brothers et al, 2013). Accumulating data indicate that priming effects on microglia might represent a key pathway to pathophysiology of depression in general and glutamatergic dysfunction, and thus have been included into our working model (Reus et al, 2015; Torres-Platas et al, 2014a, b). Microglial cells are in constant communication with astrocytes as evidenced by the dynamic interactions between these cells demonstrated in vivo (Norden et al, 2015b). Under homeostatic conditions, astrocytes respond to microglia-induced inflammatory stimulation by producing anti-inflammatory cytokines such as IL-10 and TGF-β, which act as a feedback loop to decrease microglial activation (Norden et al, 2015b, 2016). Aged astrocytes fail to restrain microglial activation probably due to a loss of or decreased surface expression IL-10 receptors in older compared with younger astrocytes (Norden et al, 2015b, 2016).
Microglial pruning
Microglia engage in constructive pruning to maintain synaptic and functional specialization during critical developmental phases and in neurogenesis (Matcovitch-Natan et al, 2016; Schafer and Stevens, 2015). In contrast, toxic pruning by microglia results from excessive immune activation and aggressive neural signaling (eg, during prenatal infections and severe stress) and appears to move the trajectory toward synaptic destruction or aberrant synaptic construction (Brites and Fernandes, 2015). Physiological synaptic pruning by microglia appear to be triggered by the expression of lipopolysaccharide-binding protein and the synaptic marker protein PSD-95 in the microglia. Decreases in the availability of these two key proteins in the microglia were noticed both in animals exposed to early life stress and prenatal infections, and the loss of these proteins was associated with aberrant synaptogenesis, deficient glutamate neurotransmission, and impaired cognitive performance (Durieux et al, 2015; Wei et al, 2012).
Microglia and glutamate
During surveillance state, microglia do not express glutamate receptors or transporters. However, upon activation, microglia express both glutamate transporters (EAAT and xC) and glutamate receptors on their surface. Glutamate binding to AMPA and kainate subtypes of ionotropic receptors located on microglia stimulates the production and release of cytokines such as TNF (Noda et al, 2000; Verkharatsky and Butt, 2013). MGluR2/3/5 is also expressed on microglia with varying modulatory effects. Glutamate binding to microglial mGluR2 promotes neurotoxicity via further release of inflammatory cytokines, glutamate, and nitric oxide (NO), whereas mGluR5 is neuroprotective through the opposing effects on same systems (Piers et al, 2011; Taylor et al, 2005). Considerable recent data implicate TNF in increasing microglial glutamate release. TNF increases extracellular glutamate levels by inducing glutamate release from microglia (Takeuchi et al, 2006) and astrocytes (Bezzi et al, 2001; Petrelli and Bezzi, 2016). The mechanism of glutamate release by microglia is linked to increased expression of the enzyme glutaminase that converts glutamine to glutamate, indicating that immune-activated glial cells actively synthesize glutamate in a manner similar to neurons (Takeuchi et al, 2008). In addition, glutamate absorbed by surface transporters of activated microglia is also trafficked into the astrocytic cytoplasm through connexin gap junctions between microglial and astrocytic cells, further leading to the decreased functioning and expression of astrocytic EAATs (Takeuchi et al, 2008; Takeuchi et al, 2006).
Microglia and mood disorders
Postmortem studies have indicated evidence of activated and primed microglia in some brain regions of depressed suicide victims including the anterior cingulate cortical regions (Torres-Platas et al, 2014a, 2014b). Recently, in vivo activation of microglia in the brains of patients with mood disorders has been imaged using positron emission tomography and a radiolabeled tracer for the translocator protein (TSPO), which is overexpressed in these cells upon activation. Using this ligand in patients with major depressive disorder, one study found increased microglial activation (Setiawan et al, 2015), whereas another did not (Hannestad et al, 2013), a discrepancy that may be related to the severity of depression, medication status, and other demographic features. Nevertheless, TSPO ligand binding reliably labeled activated glial cells following endotoxin administration in healthy human subjects (Sandiego et al, 2015). Thus, current evidence of microglial activation is variable in patients with depression; however, more work is needed in this area.
MACROPHAGES
Macrophages are recruited into the brain parenchyma through the BBB by activated resident microglia using chemokine signals including CCL2 (D’Mello et al, 2009; Le et al, 2004). In fact, many of these cells reside in perivascular regions and upon activation express glutamate transporters (EAATs and xC) and receptors (inotropic and metabotropic). In addition, they also secrete copious amounts of glutamate into the extrasynaptic space, thus adding to the excitotoxic environment of the brain during inflammation (Figure 1; Piani and Fontana, 1994; Piani et al, 1991).
T CELLS
T cells traffic into the brain through the choroid plexus and might have an important role in and confer neuroprotection by regulating glutamate and GABA neurotransmission while functioning as the cornerstone of brain’s adaptive immune system (Kipnis et al, 2012; Walsh et al, 2014b). T cells are known to decrease excitotoxicity from glutamate, modulate astroglial activity, and promote growth factor expression (Kipnis et al, 2012). Several neurotransmitters including glutamate have cognate receptors expressed on the T-cell surface that might regulate at least some of their functions including the integrin-mediated adhesion and migration in response to chemotactic signals (Ganor et al, 2003). Resting T cells also express NMDA receptors and exhibit a dose-dependent functional response to glutamate exposure (Levite, 2014). Low physiological concentrations (in nanomolar range) of glutamate appear to increase T-cell adhesion and facilitate chemotactic migration (Ganor et al, 2003). Moderately higher physiological concentrations (micromolar range) of glutamate promote T-cell proliferation via inotropic receptors (AMPA and NMDA) and decreased apoptosis via mGluRs. High concentrations (millimolar range) in a variety of pathological conditions can activate mGluR5 to decrease T-cell proliferation and mGluR1 to increase inflammatory (eg, IFN-γ) cytokine release (Ganor et al, 2003). T-cell dysfunction is one of the least explored areas in neuropsychiatry and represents a gap in our current knowledge regarding cellular regulation of glutamate neurotransmission (Ellwardt et al, 2016; Walsh et al, 2014b).
MOLECULAR MECHANISMS OF GLUTAMATE RELEASE
Synaptic release of glutamate is the primary mechanism of glutamate under physiologic conditions (Danbolt, 2001). Nevertheless, there are several important non-vesicular (non-exocytotic) mechanisms responsible for glutamate release in pathological contexts (Malarkey and Parpura, 2008; Zhou and Danbolt, 2014) including: (1) release through anion channels (Wang et al, 2013a); (2) reverse efflux through EAATs (Ye and Sontheimer, 1996, 3) release by xC transporters as part of the cystine–glutamate exchange process (Lewerenz et al, 2013, 4) astrocytic vesicular glutamate release during gliotransmission (Petrelli and Bezzi, 2016) and release through hemi-channels on astrocytes and microglia (Malarkey and Parpura, 2008). A detailed review of the functioning of these release mechanisms is beyond the scope of this review, and the reader is directed elsewhere (Dantzer and Walker, 2014; Malarkey and Parpura, 2008; Najjar et al, 2013; Tilleux and Hermans, 2007). A brief overview is presented below.
Anion Channel Glutamate Release
This mechanism of glutamate release through anion channels occurs during states of astrocytic swelling such as seen in hepatic encephalopathy and stroke. Under such conditions, glutamate is expelled by astrocytes through anion channels into the extra- and intrasynaptic spaces leading to profound excitotoxicity (Wang et al, 2013a).
Reverse Efflux of Glutamate
Astrocytes release glutamate through reverse efflux via EAATs (Malarkey and Parpura, 2008; Zhou and Danbolt, 2014). The mechanism underlying reverse release appear to involve downstream inflammatory molecules such as cyclooxygenase (COX)-2 and prostaglandin E2 both of which are known to trigger release of calcium from intracellular stores (Bezzi et al, 2001; Petrelli and Bezzi, 2016). Glutamate release by astrocytes can lead to marked ‘spillover’ effects and increase its binding to extra synaptic-NMDA receptors (Figure 1).
Glutamate Release through the Cystine–Glutamate Exchange system (xC-System)
Once thought to be exclusively astrocytic in location, now xC-transporters are known to be located on other glial cells including microglia (Lewerenz et al, 2013; Lewerenz and Maher, 2015). As described earlier, the xC-system exchanges cystine for glutamate in a 1 : 1 ratio where glutamate is extruded in exchange for intake of cystine, which is used for the synthesis of glutathione (GSH). GSH is the most abundant anti-oxidant molecule in the brain and its absence is usually fatal (Lewerenz et al, 2013; Lewerenz and Maher, 2015). Although in vitro functional ablation of the xC-system leads to fatal oxidative stress from depletion of GSH, in vivo genetic deletion of the system does not lead to oxidative stress or death, possibly due to GSH supplies from alternate cellular systems (Lewerenz et al, 2013; Lewerenz and Maher, 2015). Although the source of oxidative stress has been assumed to be due to immune stimulation, states of intense neural activity such as stress can also increase demand for GSH—which is met through an NMDA-mediated hyperactivation of GSH biosynthesis within in the neurons (Baxter et al, 2015; Nathan and Cunningham-Bussel, 2013). The glutamate that is extruded by the xC-system further adds to the extrasynaptic glutamatergic load. In several brain regions, the xC-system is a major source of both intracellular GSH and extracellular glutamate, and its enhanced transcription following induction can have widely varying effects ranging from intracellular protective effects to toxic increases in extracellular glutamate (Lewerenz et al, 2013). Excessive extracellular glutamate can bind to the xC-transporters and lead to their toxic paralysis resulting in GSH depletion and cell death—a process referred to as ‘oxidative glutamate toxicity’—in contrast to excitotoxic glutamate toxicity resulting from overstimulation of ionotropic receptors (Lewerenz et al, 2013). Thus, the combined effect of oxidative and excitotoxic glutamate toxicity has been associated with the neurotoxic effects and neurodegeneration under conditions of inflammation and possibly stress (Lewerenz and Maher, 2015).
Probably owing to their spatial proximity, glutamate released by the xC-system has preferential access to extrasynaptic NMDA receptors in a paracrine manner leading to neuronal toxicity as described earlier (Camacho and Massieu, 2006; Hardingham and Bading, 2010). Inflammatory cytokines including TNF and LPS administration upregulate the xC-system and induce glutamate release from cultured and primary microglia (Figuera-Losada et al, 2014; Mesci et al, 2015; Piani and Fontana, 1994). Similarly, IL-1β has been implicated in increasing the xC-system expression on the surface of astrocytes (but not on microglia or macrophages)—an effect that is mediated through the activation of nuclear factor kappa B (NF-kB) signaling (Jackman et al, 2010). LPS-induced activation of TLR3 and 4 increases surface expression of the xC-system and thus co-administration of LPS and the xC-system stimulator cystine can potentially lead to enhanced glutamate toxicity (Kigerl et al, 2012). Increases in xC-system function have been implicated in multiple medical disorders (Massie et al, 2015), but more intriguingly is being actively targeted for new drug development for the treatment of substance dependence (Baker et al, 2003).
Vesicular Glutamate Release
Astrocytes and oligodendrocytes sense synaptic glutamate activity through their surface glutamate receptors or transporters and respond by triggering internal Ca2+ fluxes of their own as part of the gliotransmission processes described earlier (Nedergaard et al, 2002). Oscillations in intracellular Ca2+ are known to precipitate further release of glutamate through gap junctions with other glial cells or by release into synaptic or extrasynaptic spaces (Parpura and Verkhratsky, 2013). Binding of immune stimulating molecules such as ATP and TNF to astrocytic immune receptors can amplify and alter the magnitude and propagation of intracellular Ca2+ oscillations across glial networks (Volterra and Meldolesi, 2005). Thus, exaggeration of vesicular glutamate release by glial cells by immune activation might be an important part of glutamate increases seen in mood and other disorders, although direct evidence for this is just beginning to emerge.
MOLECULAR MECHANISMS OF GLUTAMATE CLEARANCE
EAATs
EAATs are specialized transport proteins expressed on the synaptic surface of neurons and glial cells that remove and detoxify glutamate from the synaptic (and to a lesser extent extrasynaptic) space in a matter of milliseconds into the glial cells (Nedergaard et al, 2002). There are at least five different types of EAATs described with specific patterns of cellular localization. EAAT2 and EAAT1 have primarily been localized to astrocytes and oligodendrocytes, respectively, and EAAT3–4 and EAAT5 are primarily localized to neurons (Arriza et al, 1997; Danbolt, 2001). It has been estimated that almost 80% of the glutamate released by presynaptic neurons under homeostatic conditions is cleared by EAAT2 reuptake into astrocytes and up to 20% by EAAT3 into neurons (Danbolt, 2001). EAAT1 expression in oligodendrocytes suggests that EAAT1 might have a role in myelination and CNS connectivity in addition to its role in glutamate removal (Regan et al, 2007). EAATs can transport glutamate against a 10 000-fold difference in concentration gradient between intra- and extracellular compartments (Milton et al, 1997). EAATs accomplish this seemingly impossible task by pairing glutamate intake with inward transfer of three Na+ ions and one H+ ion, and outward transfer of one K+ ion from the cell, resulting in a net inward movement of positive charge (Levy et al, 1998; Zerangue and Kavanaugh, 1996).
Immune molecules act at many levels of EAAT synthesis leading to a decrease in EAAT synthesis, expression, and functionality. Most importantly, the EAAT genes have promoter regions that are responsive to immune regulation through intracellular immune signaling molecules such as the transcription factor NF-kB (Zou and Crews, 2005). EAAT proteins are synthesized in the endoplasmic reticulum and appear to undergo extensive posttranslational modification in the Golgi apparatus (Kalandadze et al, 2004) before being trafficked onto the plasma membrane for surface expression (Conradt and Stoffel, 1997; Figiel and Dzwonek, 2007; Figiel et al, 2007; Gamboa and Ortega, 2002; Schluter et al, 2002; Shan et al, 2013). The surface EAATs are periodically removed from the plasma membrane via endocytosis, and are then either shuttled back to the cell surface (via recycling endosomes) or targeted for degradation in lysosomes (Nakagawa et al, 2008). The entire process is regulated by protein–protein interactions and phosphorylation, and is highly responsive to immune influences. Immune mediators (such as TNF) regulate membrane trafficking and recycling of EAATs either alone or in association with several neurohumoral influences, including growth factors and neuropeptides (Gras et al, 2012; Takahashi et al, 2015). As mentioned previously, EAATs are expressed on astrocytes and oligodendrocyte cells both in resting and during activation states, whereas microglia express these transporters only when activated by immune factors such as LPS (Persson et al, 2005). Inflammatory molecules such as IL-1β and TNF released during immune activation suppress astrocytic expression of GLT-1 and GLAST glutamate transporters (similar to EAAT2 and EAAT1 in humans) in cell culture experiments (Korn et al, 2005; Szymocha et al, 2000; Wang et al, 2013a; Ye and Sontheimer, 1996), and EAAT2 expression is diminished in inflammatory CNS lesions of both laboratory animals and humans (Ohgoh et al, 2002; Vercellino et al, 2007). Several excellent reviews on this topic are available for further reference (Malarkey and Parpura, 2008; Ohgoh et al, 2002; Tavares et al, 2002; Tilleux and Hermans, 2007; Vercellino et al, 2007). Although glutamate removal from the extracellular space by EAATs is sufficient to prevent excitotoxicity from physiological levels of glutamate release (Bergles and Jahr, 1997; Rothstein, 1996), it is often inadequate in pathological contexts such as during immune activation (Danbolt, 2001; Dantzer and Walker, 2014; Zhou and Danbolt, 2014).
BBB Glutamate Scavenging
Brain extracellular levels of glutamate are normally maintained in the micromolar range by EAATs but can rapidly increase 100-fold during microglial and macrophage release (Dantzer and Walker, 2014; Leibowitz et al, 2012). However, when glutamate concentrations become excessive through contributions from activated microglia and macrophages, the EAAT uptake mechanism may be insufficient to clear the entire glutamate load with further impairment of glutamate clearance (Gras et al, 2012; Takaki et al, 2012). Glutamate is also removed by passive diffusion from the brain through the BBB into the systemic circulation. Extracellular glutamate effluxes through astrocytes into the perivascular space and then through the endothelium into the peripheral blood. It is now known that much of extracellular glutamate is removed by EAATs expressed on the abluminal (brain side) of the endothelium of the cerebral blood vessels, crosses the blood vessel wall, and is released into the blood by glutamate transporters present on the luminal or blood side of the endothelium (Helms et al, 2012). Thus, rapid reduction in the concentrations of plasma glutamate leads to an aggressive mobilization of glutamate from the brain into the plasma and can result in rapid reduction of brain glutamate. This in turn can be clinically accomplished by administering agents such as oxaloacetate and pyruvate that stimulate glutamate-metabolizing enzymes such as glutamate oxaloacetate and pyruvate transaminase (SGOT and SGPT) in the blood and lead to rapid depletion of blood glutamate. This strategy has met with some success in reducing glutamate-induced excitotoxicity following experimental induction of stroke (Leibowitz et al, 2012). More recently, this strategy of glutamate ‘scavenging’ has been proposed as a possible means to reduce excessive glutamate activity as a result of increased inflammation (Dantzer and Walker, 2014).
CYTOKINE EFFECTS ON GLUTAMATE NEUROTRANSMISSION
There is a rich literature on the impact of multiple cytokines on glia and glutamate neurotransmission including the cellular expression of glutamate receptors, and glutamate release and reuptake (Vezzani and Viviani, 2015). Selected effects of several inflammatory cytokines are reviewed below and are summarized in Table 1.
TNF
In vitro studies of primary hippocampal neurons have shown that TNF signaling controls synaptic scaling by increasing trafficking of AMPA glutamate receptors and decreasing the numbers of surface GABA receptors (Wohleb et al, 2016). Experimental application of TNF to hippocampal slices induced a rapid increase in excitatory postsynaptic electrical activity mediated by increased expression of overactive surface AMPA receptors (Beattie et al, 2002) associated with an overrepresentation of GluA1compared with GluA2subunits (Vezzani and Viviani, 2015). The hyperactive AMPA subtype is known to extra-permeable to Ca2+ leading to acute enhancement of Ca2+ rises and has been associated with neuroinflammation-induced disease progression in epilepsy (Vezzani and Viviani, 2015). Effects of TNF on NMDA receptors is not yet well defined, although NMDA receptor blockers are known to confer neuroprotection against glutamate toxicity resulting from TNF-mediated EAAT inhibition (Zou and Crews, 2005). Thus, at physiological concentrations, TNF might promote synaptogenesis by stimulating postsynaptic AMPA activity (Pribiag and Stellwagen, 2014), synaptic maintenance by optimally stimulating pre- and postsynaptic NMDA through the TNFR1 system (Santello et al, 2011), regulate EAAT function to promote controlled clearance of glutamate (Volterra and Meldolesi, 2005), and increase neuroprotection through TNFR2 activity. (Cheng et al, 1994) However, during immune activation, TNF levels can rapidly increase from picomolar and micromolar levels to as high as 100 mM levels leading to sustained NF-kB activation and neurotoxicity (Santello and Volterra, 2012).
IL-1β
Hippocampal cell culture studies suggest that the activation of NMDA receptors promote membrane insertion of new IL-1β receptors, whereas IL-1β enriches the NMDA receptor pool and enhances its activity at the postsynaptic membrane (Vezzani and Viviani, 2015). By preferentially targeting the NMDA receptor complex, IL-1β is hypothesized to lead to neuroprogression, kindling, and enhanced risk of excitotoxicity by promoting downstream consequences including tyrosine-kinase (TrK)-mediated phosphorylation of NMDA receptor proteins and progressively increasing Ca2+ permeability with repeated activation (Vezzani and Viviani, 2015). IL-1β release may be mediated by the activation of NLRP3-inflammasome systems by DAMPs (such as high-mobility box group (HMGB)-1) and ATP; Vezzani and Viviani, 2015) and it is activated by stress (Iwata et al, 2013; Iwata et al, 2016). Taken together, the data indicate the marked engagement of progressive NMDA activation by IL-1β signaling continues to represent a target for neuroprogressive changes in mood disorders.
IFN-γ
IFN-γ activation can lead to profound changes in the functioning of astrocytes, thus leading to changes in their ability to clear glutamate. Unlike other cytokines, T lymphocytes are the primary producers of IFN-γ (Kipnis et al, 2012) and once released at high concentrations, IFN-γ can transform astrocytes into phagocytic, antigen-presenting cells (Fontana et al, 1984; Shrikant and Benveniste, 1996; Soos et al, 1998). In concert with the anti-inflammatory cytokine IL-4 (which is also produced by T cells), IFN-γ controls the transition of the astroglial phenotype from neuroprotective (A2) to neurotoxic (A1) with the drastic reduction in the ability of these cells to clear glutamate (Garg et al, 2008; Garg et al, 2009; Kipnis et al, 2012). However, at lower concentrations, IFN-γ increases glutamate buffering and clearance by stimulating EAATs with the opposite effects at higher concentrations (Hu et al, 2000; Ye and Sontheimer, 1996). It also serves as a potent inducer of the tryptophan-catabolizing enzyme indoleamine-2,3-dioxygenase (IDO) in microglia ultimately leading to the generation of glutamate-like compounds such as QUIN further altering glutamate neurotransmission (Oxenkrug, 2011; Oxenkrug, 2010). IFN-γ may induce neuronal dysfunction by the enhancement of AMPA (but not NMDA)-mediated glutamate neurotoxicity (Mizuno et al, 2008).
IL-6
IL-6 is a key component of the inflammatory response in the brain, a profound stimulator of acute phase proteins from the liver and has been implicated in the etiology of neuropsychiatric disorders such as depression (Dowlati et al, 2010; Howren et al, 2009; Miller and Raison, 2016; Valkanova et al, 2013) and schizophrenia (Goldsmith et al, 2016). IL-6 has mixed effects on the glutamate system attributable to the more chronic and nuanced nature of its effects. Unlike IL-1β and TNF, IL-6 is secreted mostly by astrocytes and its effects are mediated through its soluble receptor (sIL-6R), which acts on cells expressing gp130 on their surface (Arisi, 2014). IL-6 leads to decreases in presynaptic glutamate release, immediate but transient enhancement of AMPA activity followed by its later decline and downregulation of NMDA receptor expression and activity (Pribiag and Stellwagen, 2014). In sum, IL-6 effects on glutamate metabolism and turnover is varied but more study is warranted.
DUAL ACTIVATION OF INFLAMMATION AND GLUTAMATE RELEASE: P2X7 RECEPTORS
So far, we have reviewed mechanisms by which the immune system and inflammation might lead to increases in glutamate concentrations. However, both the immune system and astrocytic glutamate release can be activated in concert by purinergic mechanisms stimulated by extracellular release of ATP (Li et al, 1999; Longuemare and Swanson, 1995; Rossi et al, 2000; Zeevalk et al, 1998). ATP is released into the extracellular space by apoptotic dead tissue or stress-induced neuronal firing and can rapidly activate the inflammasome, leading to the release of cytokines such as IL-1β and IL-18 (de Rivero Vaccari et al, 2014, 2016a, 2016b). In addition to immune effects, binding of ATP to purinergic P2X7 cation channel receptors on glial cells is also known to trigger direct release of glutamate through these channels (Malarkey and Parpura, 2008). In contrast to other astrocytic ion hemichannels, P2X7 channels are insensitive to voltage changes and quickly become conduits for the reverse release of glutamate from astrocytes in a calcium-independent manner (Malarkey and Parpura, 2008). The leakage of glutamate through P2X7 ion channels can lead to drastic increases in glutamate. Intriguingly, a recent in vivo study using immobilization stress (a preclinical model of mood disorder) reported that concurrent stress-induced increases in the release of both glutamate (from neurons) and ATP (from astrocytes) was followed by the stimulation of the microglial inflammasome apparatus leading to release of IL-1β (Iwata et al, 2016). Based on this data, the acute neurotoxic effects of ATP might be mediated through P2X7 channel release of glutamate, with more chronic effects being mediated through activation of inflammasome.
METABOLIC MECHANISMS OF GLUTAMATE PATHWAY ACTIVATION
KYN Pathway
Tryptophan is an essential amino-acid available primarily through dietary intake and has multiple biological roles such as being a substrate in the synthetic pathway of the mood regulating monoamine serotonin. During inflammatory conditions, tryptophan is shunted away from serotonin synthesis into the KYN pathway, which is an alternative catabolic pathway for tryptophan resulting in the generation of multiple glutamate-like metabolites that can trigger neurotoxicity and oxidative stress (Dantzer and Walker, 2014; Schwarcz, 2016). The metabolism of tryptophan through the KYN pathway is initiated by activation of tryptophan-catabolizing enzymes IDO-1 and 2, and tryptophan-2,3-dioxygenase (TDO) (Schwarcz, 2004, 2016; Schwarcz et al, 2012; Schwarcz and Pellicciari, 2002). TDO is found in the liver and is regulated by glucocorticoids, whereas IDO is found both in the periphery and in the brain immune cells, and it is responsive to inflammatory cytokines including IFN-γ and TNF (Dantzer et al, 2008; Schwarcz et al, 2012). Once activated, the IDO system leads to a sequence of multi-step, enzyme-substrate reactions, which culminate in the generation of a wide range of glutamate-like and free radical-generating metabolites (Stone et al, 2012). Robust evidence has linked IDO activation to symptoms of depression following administration of IFN-α for the treatment of cancer and hepatitis C (Capuron et al, 2003; Raison et al, 2010). Taken together, the KYN pathway involves metabolites whose primary effects may be mediated through glutamatergic systems and hence might represent pharmacological targets to modulate glutamatergic neurotransmission. Given the vast number or review articles available on this subject (Dantzer et al, 2011; Myint et al, 2007; Oxenkrug, 2010; Schwarcz, 2016; Schwarcz et al, 2012; Stone et al, 2012; Vecsei et al, 2013), only topics essential to glutamate activity will be discussed in the ensuing paragraphs.
KYN Pathway and its Downstream Metabolites
The KYN pathway is initiated by the transformation of tryptophan to N-formylkynurenine by IDO, which in turn is converted into KYN by formamidase (Schwarcz et al, 2012). Approximately 60% of KYN in the brain are synthesized in the peripheral tissues and enters the brain through amino-acid uptake transporters in the BBB and the remaining 40% are synthesized de novo in the brain by IDO in activated immune and glial cells (Vecsei et al, 2013). Generation of KYN is a key step in the initiation of the KYN pathway (Schwarcz et al, 2012). Irreversible transamination of KYN by action of the enzyme kynurenine aminotransferase II results in the generation of an NMDA antagonist kynurenic acid (KYNA) with putative neuroprotective properties. Indeed, KYNA is a robust antagonist of α-7 nicotinic acetylcholine receptors (α7nAchR), which leads to secondary decreases in presynaptic glutamate release (Schwarcz et al, 2012). Sequential activation by the enzymes kynurenine monooxygenase (KMO) and kynureninase results in the formation of the neurotoxic molecules 3-hydroxy kynurenine (3HK), anthranilic acid (AA), 3-hydroxy anthranilic acid (HANA), and QUIN that are ultimately converted to nicotinic acid adenine dinucleotide (NAD). 3HK, AA and, HANA all are known to trigger profound oxidative stress and precipitate cell death (Chiarugi et al, 2001a; Chiarugi et al, 2001b; Guidetti and Schwarcz, 1999). They also indirectly contribute to glutamate increases by increasing the need for GSH and activating the xC-system. QUIN is a direct-acting NMDA receptor agonist and powerful neurotoxin (Guillemin, 2012).
The balance between the concentrations of the various KYN byproducts depends on the activation status of the cells that host the synthetic enzyme systems for these molecules. The KYNA-synthesizing enzyme KAT is only present in astrocytes. Hence, the quantity of KYNA is dependent on astrocytic health and functioning (Schwarcz et al, 2012). In stark contrast, the enzyme KMO is present only in microglia, and its activation leads to increases in concentrations of neurotoxic metabolites including 3HK, AA, HANA, and QUIN (Schwarcz et al, 2012). Dysregulation of either of these systems or a loss of the balanced function of these systems is hypothesized to contribute to the pathogenesis and pathophysiology of several neuropsychiatric disorders. For example, increases in KYNA activation are associated with schizophrenia, whereas QUIN increases are associated with depression, suicidality, and neurodegeneration (Brundin et al, 2015; Erhardt et al, 2013; Guillemin, 2012; Savitz et al, 2015; Schwarcz et al, 2012).
QUIN Effects on Glutamate
QUIN is a powerful and potent excitotoxin, and the prolonged presence of even physiological concentrations (mid-to-high nanomolar range) of QUIN is sufficient to cause excitotoxicity (Guillemin, 2012; Schwarcz, 2016). Short-term exposure of neurons to QUIN can cause rapid neuronal excitation by activating NMDA receptors (Guillemin, 2012). QUIN selectively induces greater activation of NMDA receptors in neurons located in hippocampus, striatum, and neocortex, leading to a higher excitotoxic burden in these brain regions (Guillemin, 2012; Tavares et al, 2002). Mechanistically, it is proposed that differences in NMDA receptor configuration in these regions might mediate their hyper-responsiveness to QUIN toxicity (Dantzer et al, 2008; Guillemin, 2012; Schwarcz, 2004, 2016; Schwarcz et al, 2012). QUIN toxicity is often additive or synergistic with concurrently present neurotoxic processes including immune activation, oxidative stress, and excitotoxicity (Guillemin, 2012).
QUIN Effects on Glia
QUIN is also able to cause gliotoxicity (Guillemin, 2012). At low concentrations, QUIN treatment induced astrocytic activation, decreased EAAT activity, and produced a dose-dependent reduction in glutamine synthetase activity resulting in a net increase in tissue glutamate concentrations (Schwarcz, 2016). The mechanism of action of QUIN on astrocytes is likely to be mediated via activation of astrocytic NMDA receptors, which may be preferentially sensitive to the NMDA receptor ion channel blockers MK801 and memantine offering some hope for protection from QUIN toxicity in diseases such as Huntington’s disease (Guillemin, 2012; Schwarcz, 2016), as well as other disorders of increased inflammation and behavioral dysregulation.
CONCEPTUAL CONVERGENCE: A WORKING MODEL
So far, we have reviewed the rich literature describing how activated immune mechanisms impact and alter cellular, molecular, and metabolic mechanisms that regulate glutamate in both physiological and pathological contexts. Based on the above data, we propose a working model that implicates dysregulation of glutamate processing by glial and neuronal cells resulting from inflammatory activation as a causal factor mediating behavioral changes in some patients with mood disorders (Figure 1). The model also proposes to incorporate early and late effects of inflammatory activation on glutamate processing by neurons, astrocytes, oligodendrocytes, and microglia leading to neuroprogressive changes often seen in mood disorders (Moylan et al, 2013).
Immune-induced changes in astrocytes leading to both defective cradling and impaired buffering by EAATs are proposed to result in defective containment and spillover of neuronal glutamate into the extrasynaptic regions (Haydon and Carmignoto, 2006; Parpura and Verkhratsky, 2013; Volterra and Meldolesi, 2005). Non-neuronal release of glutamate by activated and/or primed microglia, macrophages and astrocytes through xC system, hemichannel, reverse EAAT, anion channel and vesicular release mechanisms further contribute to increased glutamate available for diffusion into the extrasynaptic space (Ida et al, 2008; Malarkey and Parpura, 2008; Tilleux and Hermans, 2007). Of note, the effects of priming might have profound impact on the release of glutamate by microglial cells and might also impact the antidepressant effects of glutamatergic modulating agents.
The increased extrasynaptic diffusion of glutamate resulting from the combined effects of spillover and glial release further exerts downstream effects on extrasynaptic glutamate receptors. The extent and effects of glutamate diffusion will depend upon the spatial arrangement of extrasynaptic glutamate receptors, glutamate synapses, and their glutamate transporter buffering zones (Dorsett et al, 2016; McCullumsmith and Sanacora, 2015; Tzingounis and Wadiche, 2007). In physiological conditions, the buffering and release of glutamate is tightly regulated by EAATs and glutamate is not allowed to diffuse beyond 0.5 μm from its point of origin, thus restricting access to extrasynaptic glutamate receptors (Tzingounis and Wadiche, 2007). It is possible that decreases in number and functioning of EAATs by immune molecules drastically decreases buffering zones, increases the radius of spillover, and increases availability of freely diffusing glutamate to engage and over activate extrasynaptic binding sites in a paracrine manner (Tzingounis and Wadiche, 2007).
Extrasynaptic NMDA receptor stimulation has been implicated as one of the primary culprits mediating the early and late effects of glutamate toxicity by blocking the synthesis and release of nerve growth factors such as BDNF and other synaptic proteins resulting in synaptic loss (Hardingham, 2009; Hardingham and Bading, 2010; Hardingham et al, 2002). Extracellular diffusion of glutamate can lead to the loss of synaptic fidelity, decreased specificity of neurotransmission, and lead to ‘noisy’, non-specific, and disruptive neural activity experienced by patients as cognitive and affective symptoms (Dorsett et al, 2016). Although precise data are lacking, we speculate that inflammatory activation in mood disorders might lead to increased AMPA activity during early phases followed by desensitization and decreased AMPA activity in later stages resulting in synaptic depression, decreased transmission efficiency, and impaired synaptic plasticity (Choquet and Triller, 2013; Popoli et al, 2012; Pribiag and Stellwagen, 2014; Pribiag and Stellwagen, 2013).
Increased extrasynaptic glutamate can also bind to presynaptic neuronal metabotropic mGluR2/3 autoreceptors inhibiting glutamate release leading to chronic reductions in intrasynaptic glutamate, loss of excitatory synaptic activity, and ultimately result in synaptic loss (Duman, 2014; Duman et al, 2016; McEwen et al, 2016). Of note, mGluR2/3 antagonists have demonstrated ketamine-like antidepressant effects (Duman, 2014; McEwen et al, 2016). Finally, we propose that the decrease in intrasynaptic glutamate also leads to further atrophy and loss of intrasynaptic NMDA receptors. The resulting imbalance between decreases in intrasynaptic vs increases in extrasynaptic NMDA signaling is hypothesized to lead to neurotoxicity, neuronal loss, and decreased regional brain volumes seen in patients with mood disorders (Sanacora and Banasr, 2013; Wang et al, 2013b). During gliotransmission, the astrocytes release small amounts of glutamate that binds to extrasynaptically located presynaptic glutamate receptors that can increase or decrease synaptic release—thus tuning synaptic activity up or down (De Pitta and Brunel, 2016). We speculate that processes mediating successful treatments or spontaneous remission in mood disorders engage these astroglia-mediated plasticity and repair mechanisms to reverse synaptic dysfunction. In accordance with the previously published literature, we propose that this model implicates the role played by glial cells in mediating the convergence between inflammation and glutamate seen among a number of intermediate behavioral phenotypes, including positive (reward learning and reward valuation) and negative (fear, anxiety and loss) valence systems, cognition, arousal, and socialization (Haroon et al, 2012, 2016; McCullumsmith and Sanacora, 2015).
PRECLINICAL AND CLINICAL STUDIES: TRANSLATIONAL RELEVANCE
Based on the multitude of interactions among the immune system, glia, and glutamate metabolism as noted in our working model, several recent preclinical and clinical studies have examined the association between inflammation and glutamate dysregulation. For example, blockade of glutamate by pre-administration of ketamine abrogated depressive symptoms following LPS without altering underlying inflammatory responses in the brain (Walker et al, 2013). Moreover, in an animal model of treatment refractory depression using chronic administration of ACTH, animals that responded to ketamine exhibited higher baseline plasma concentrations of CRP and TNF (Walker et al, 2015). Elevated peripheral blood TNF, IL-1β, and IL-6 predicted favorable antidepressant response to ketamine administration in a small sample of depressed patients (Hashimoto, 2015). Other studies report that increased body mass index (BMI) and plasma adipokine concentrations (both of which are associated with inflammation) correlated with ketamine response (Jaso et al, 2016; Machado-Vieira et al, 2016; Machado-Vieira et al, 2009). Intriguingly, as noted above, astrocytes express adipokine receptors such as the leptin receptors (LepR) and LepRs are increased in number in the astrocytes of obese animals and in C6 astrocytoma cells treated with LPS and TNF (Hsuchou et al, 2009; Pan et al, 2012).
More directly, addressing the impact of inflammation on glutamate, creatine-normalized glutamae (Glu/Cr) concentrations in the basal ganglia and anterior cingulate cortex (dACC) were measured using proton magnetic resonance spectroscopy (MRS) before and after administration of IFN-α (Haroon et al, 2014). IFN-α led to significant increases in Glu/Cr in the left basal ganglia and the dACC, which in turn correlated with depressive symptoms (Haroon et al, 2014). Of relevance to the potential role of the KYN pathway in these findings, is that IFN-α increases QUIN in the CSF of IFN-α-treated patients in association with depressive symptoms (Raison et al, 2010). Another study showed that IFN-α-induced Glu/Cr increases were seen more robustly among older compared to younger individuals and these Glu/Cr increases were associated with greater plasma TNF concentrations, increased psychomotor slowing and decreased motivation (Haroon et al, 2015). In sum, these findings indicate a greater susceptibility of glutamate metabolism to the effect of inflammation among older individuals (Haroon et al, 2015). These findings may be related to the alterations in glia function as a result of priming effects of aging described above.
Further substantiating the relationship between increased inflammation (as reflected by CRP) and glutamate, chronically depressed patients with varying levels of inflammatory activation underwent MRS to evaluate absolute glutamate concentrations in the left basal ganglia and the dACC (Haroon et al, 2016). Patients with major depression and high inflammatory activity (plasma CRP>3 mg/l) showed significantly higher concentrations of glutamate in the left basal ganglia region compared to patients with low inflammation (CRP<1 mg/l; Haroon et al, 2016). In addition, a significant correlation was found between plasma and CSF CRP, and basal ganglia glutamate. No glutamate changes as a function of inflammation were seen in the dACC of these depressed patients (Haroon et al, 2016). Of note, inflammation-induced basal ganglia glutamate changes were associated with behavioral changes including anhedonia and psychomotor slowing (Haroon et al, 2016). Interestingly, aside from glutamate, the only other neurometabolite associated with inflammation both in the peripheral blood and CSF was myo-inositol (Haroon et al, 2016). Myo-inositol is believed to be an astroglial marker, suggesting an involvement of astrocytes in these findings (Brand et al, 1993; Haroon et al, 2009; Kim et al, 2005; Plitman et al, 2016). Taken together, these data indicate an association between inflammation and glutamate dysregulation in mood disorders including depression. Moreover, these findings are consistent with the rich basic science data demonstrating the impact of inflammation on the cellular, molecular, and metabolic constituents involved in the regulation of glutamate metabolism. Finally, these data identify potentially relevant biomarkers of inflammation effects on glutamate metabolism in mood disorders including peripheral blood inflammatory measures such as CRP, TNF, and IL-6; KYN metabolites such as QUIN; adipokines; and other markers of altered metabolic function including BMI, and central markers of altered glial function such as increased myo-inositol.
Regarding treatment considerations, as discussed above, the NMDA antagonist ketamine has received the most attention regarding glutamate alterations in mood disorders and may have special efficacy in patients with increased inflammation. Nevertheless, other glutamate active drugs warrant consideration. For example, memantine—a non-competitive but weak NMDA receptor antagonist—administered at doses ranging from 5 to 20 mg per day was not associated with antidepressant efficacy in controlled trials (Niciu et al, 2014a). All studies used doses of memantine approved for the treatment of dementia and it is unclear whether higher doses of memantine might have yielded better results. However, no study has used inflammatory biomarkers to predict memantine response, as with ketamine. Riluzole, a glutamate-stabilizing agent that acts by increasing uptake of glutamate into the glial cell and by modulating release of presynaptic glutamate has proven effective in reducing the physiological, cellular, and behavioral effects of chronic stress in rodent models (Banasr et al, 2010). Several small open-label clinical studies have also shown some promise for the drug in the treatment of mood and anxiety disorders (Pittenger et al, 2008). However, data from randomized placebo-controlled trials have been mixed, although none of these studies examined inflammatory biomarkers as potential predictors of response. A single parallel, randomized, controlled trial, study of 64 inpatients with diagnosis of moderate-to-severe major depressive disorder from Iran found that the patients receiving 6 weeks of treatment with riluzole (50 mg/bid) plus citalopram (40 mg per day) had a significantly greater response with greater speed to treatment was observed in the riluzole group than the placebo plus citalopram (40 mg per day) group (Salardini et al, 2016), but other studies have failed to show any clear evidence to support the efficacy of the drug in the treatment of major depressive disorder (Mathew et al, 2015; Niciu et al, 2014b). Similar to memantine, no study has taken into consideration the inflammatory status of the sample population. Several proprietary drugs with effects on NMDA systems are under varying stages of development and evaluation, and the reader is referred to other more in depth articles focusing on this topic (Niciu et al, 2014a; Sanacora et al, 2012; Zarate et al, 2010).
CONCLUSIONS AND FUTURE DIRECTIONS
Extensive data indicate that the immune system has multiple roles in the regulation of glutamate neurotransmission and the maintenance of synaptic integrity. In addition, glutamate can profoundly influence the function of immune cells in the brain including microglia. Relevant to pathology, increased activation of the immune response in the periphery and CNS can lead to marked alterations in glial function and glutamate regulation, and through the activation of multiple avenues of excitotoxicity can lead to synaptic dysfunction and death, and ultimately behavioral and cognitive impairments. Much interest has focused on the role of these interactions among inflammation, glutamate metabolism, and glial function in mood disorders. However, studies have failed to fully establish whether inhibition of inflammation can reverse CNS glutamate changes as assessed by neuroimaging strategies such as MRS. Moreover, it remains unclear whether and which markers of inflammation might identify those patients who are most likely to respond to glutamate-targeted therapies. In addition, the contribution of inflammation-induced changes in glutamate to white matter pathology remains unclear. Finally, further examination of how treatments that target inflammation and glutamate can be combined or sequenced to achieve lasting response or disease prevention is warranted. In summary, it has become increasingly clear that there are many more pathways to pathology in mood and other neuropsychiatric disorders that go beyond the monoamines. Inflammation and its effect on glia and glutamate are two of these pathways, and data indicate that there is an important convergence that if recognized and studied may greatly enhance the further development of therapeutics that target these pathways.
FUNDING AND DISCLOSURE
This work was supported by funds from the National Institute of Mental Health to EH (R01MH107033, K23MH091254, and NARSAD), AHM (R01MH087604 and R03MH100273), and GS (National Center for PTSD—VA CT Healthcare System, DMHAS, CT, and NARSAD). Dr Sanacora has received consulting fees form Allergan, Alkermes, AstraZeneca, BioHaven Pharmaceuticals, Hoffman La-Roche, Janssen, Merck, Naurex, Servier Pharmaceuticals, Taisho Pharmaceuticals, Teva, and Valenant pharmaceutical North America, Vistagen therapeutics over the last 24 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Johnson & Johnson, Hoffman La-Roche, Merck & Co., Naurex, and Servier over the last 24 months. Free medication was provided to Dr Sanacora for an NIH sponsored study by Sanofi-Aventis. In addition, he holds shares in BioHaven Pharmaceuticals Holding Company and is a co-inventor on a patent ‘Glutamate agents in the treatment of mental disorders’, patent number: 8778979. The remaining authors declare no conflict of interest.
References
Abbott NJ, Ronnback L, Hansson E (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53.
Allam SL, Bouteiller JM, Hu EY, Ambert N, Greget R, Bischoff S et al (2015). Synaptic efficacy as a function of ionotropic receptor distribution: a computational study. PLoS One 10: e0140333.
Allam SL, Ghaderi VS, Bouteiller JM, Legendre A, Ambert N, Greget R et al (2012). A computational model to investigate astrocytic glutamate uptake influence on synaptic transmission and neuronal spiking. Front Comput Neurosci 6: 70.
Arisi GM (2014). Nervous and immune systems signals and connections: cytokines in hippocampus physiology and pathology. Epilepsy Behav 38: 43–47.
Arriza JL, Eliasof S, Kavanaugh MP, Amara SG (1997). Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA 94: 4155–4160.
Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S et al (2003). Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–749.
Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL et al (2010). Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15: 501–511.
Banasr M, Duman RS (2008). Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry 64: 863–870 This is an important paper establishing that glial ablation in the PFC is sufficient to induce depressive-like behaviors similar to chronic stress and depressive symptoms.
Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS (2007). Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry 62: 496–504.
Barley K, Dracheva S, Byne W (2009). Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 112: 54–64.
Barres BA (2008). The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60: 430–440.
Bartzokis G (2004). Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 25: 5–18.
Baxter PS, Bell KF, Hasel P, Kaindl AM, Fricker M, Thomson D et al (2015). Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat Commun 6: 6761.
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M et al (2002). Control of synaptic strength by glial TNFalpha. Science 295: 2282–2285 This paper was one of the earliest to demonstrate that cytokines have neuroactive properties and can affect synaptic modeling.
Bechtholt-Gompf AJ, Walther HV, Adams MA, Carlezon WA Jr, Ongur D, Cohen BM (2010). Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 35: 2049–2059.
Bergles DE, Jahr CE (1997). Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19: 1297–1308.
Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD et al (2011). Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16: 634–646.
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E et al (2001). CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci 4: 702–710 This is the one of the earliest papers to propose a new pathway for glia-glia and glia-neuron communication that is relevant to both normal brain function and neurodegenerative diseases.
Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ et al (2015). Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 88: 941–956.
Bowley MP, Drevets WC, Ongur D, Price JL (2002). Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry 52: 404–412.
Brand A, Richter-Landsberg C, Leibfritz D (1993). Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15: 289–298.
Braun K, Antemano R, Helmeke C, Buchner M, Poeggel G (2009). Juvenile separation stress induces rapid region- and layer-specific changes in S100ss- and glial fibrillary acidic protein-immunoreactivity in astrocytes of the rodent medial prefrontal cortex. Neuroscience 160: 629–638.
Brites D, Fernandes A (2015). Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci 9: 476.
Brothers HM, Bardou I, Hopp SC, Kaercher RM, Corona AW, Fenn AM et al (2013). Riluzole partially rescues age-associated, but not LPS-induced, loss of glutamate transporters and spatial memory. J Neuroimmune Pharmacol 8: 1098–1105.
Brundin L, Erhardt S, Bryleva EY, Achtyes ED, Postolache TT (2015). The role of inflammation in suicidal behaviour. Acta Psychiatr Scand 132: 192–203.
Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192.
Camacho A, Massieu L (2006). Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res 37: 11–18.
Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D et al (2003). Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry 54: 906–914.
Chen X, Errangi B, Li L, Glasser MF, Westlye LT, Fjell AM et al (2013). Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and microstructural changes. Neurobiol Aging 34: 2248–2260.
Cheng B, Christakos S, Mattson MP (1994). Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron 12: 139–153.
Chiarugi A, Calvani M, Meli E, Traggiai E, Moroni F (2001a). Synthesis and release of neurotoxic kynurenine metabolites by human monocyte-derived macrophages. J Neuroimmunol 120: 190–198.
Chiarugi A, Meli E, Moroni F (2001b). Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J Neurochem 77: 1310–1318.
Choquet D, Triller A (2013). The dynamic synapse. Neuron 80: 691–703.
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C et al (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504: 394–400.
Conradt M, Stoffel W (1997). Inhibition of the high-affinity brain glutamate transporter GLAST-1 via direct phosphorylation. J Neurochem 68: 1244–1251.
Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L et al (2002). The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry 51: 377–386.
Cotter DR, Pariante CM, Everall IP (2001). Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 55: 585–595.
Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006). Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31: 1616–1626.
D’Mello C, Le T, Swain MG (2009). Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci 29: 2089–2102.
Danbolt NC (2001). Glutamate uptake. Prog Neurobiol 65: 1–105 A primary reference paper explicating the primary role played by glutamate transporters are in almost all aspects of normal and abnormal brain activity.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56 This review summarizes three decades of work by the authors on the overlap between sickess behavior and symptoms of depression. Establishes inflammation as an important biological event that increases the risk of major depressive episodes similar to psychosocial factors.
Dantzer R, O’Connor JC, Lawson MA, Kelley KW (2011). Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36: 426–436.
Dantzer R, Walker AK (2014). Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J Neural Transm 121: 925–932.
De Pitta M, Brunel N (2016). Modulation of synaptic plasticity by glutamatergic gliotransmission: a modeling study. Neural Plast 2016: 7607924.
de Rivero Vaccari JP, Brand F 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY et al (2016a). Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem 136 (Suppl 1): 39–48.
de Rivero Vaccari JP, Dietrich WD, Keane RW (2014). Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab 34: 369–375.
de Rivero Vaccari JP, Dietrich WD, Keane RW (2016b). Therapeutics targeting the inflammasome after central nervous system injury. Transl Res 167: 35–45.
Dilger RN, Johnson RW (2008). Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol 84: 932–939.
Dimou L, Gallo V (2015). NG2-glia and their functions in the central nervous system. Glia 63: 1429–1451.
Domercq M, Sanchez-Gomez MV, Sherwin C, Etxebarria E, Fern R, Matute C (2007). System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol 178: 6549–6556.
Dorsett CR, McGuire JL, DePasquale EA, Gardner AE, Floyd CL, McCullumsmith RE (2016). Glutamate neurotransmission in rodent models of traumatic brain injury. J Neurotrauma (e-pub ahead of print; doi:10.1089/neu.2015.4373). A recently published review that provides detailed descriptions of the dysregulation of extracellular glutamate, glutamate uptake changes in the acute stage of TBI and its role in chronic cognitive symptoms after TBI.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al (2010). A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457.
Duman RS (2014). Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci 16: 11–27.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22: 238–249.
Duman RS, Voleti B (2012). Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35: 47–56.
Durieux AM, Fernandes C, Murphy D, Labouesse MA, Giovanoli S, Meyer U et al (2015). Targeting glia with N-acetylcysteine modulates brain glutamate and behaviors relevant to neurodevelopmental disorders in C57BL/6J mice. Front Behav Neurosci 9: 343.
Edgar N, Sibille E (2012). A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry 2: e109 This article provides an excellent model, to demonstrate that impaired oligodendrocyte structure and function impacts neural circuitry leading to downstream effects related to emotionality in rodents and patients with mood disorders.
Eggen BJ, Raj D, Hanisch UK, Boddeke HW (2013). Microglial phenotype and adaptation. J Neuroimmune Pharmacol 8: 807–823.
Ellwardt E, Walsh JT, Kipnis J, Zipp F (2016). Understanding the role of T cells in CNS homeostasis. Trends Immunol 37: 154–165.
Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M et al (2013). Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 38: 743–752.
Ernst C, Deleva V, Deng X, Sequeira A, Pomarenski A, Klempan T et al (2009). Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch Gen Psychiatry 66: 22–32.
Estes ML, McAllister AK (2015). Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 16: 469–486.
Evonuk KS, Baker BJ, Doyle RE, Moseley CE, Sestero CM, Johnston BP et al (2015). Inhibition of system Xc(-) transporter attenuates autoimmune inflammatory demyelination. J Immunol 195: 450–463.
Farina C, Aloisi F, Meinl E (2007). Astrocytes are active players in cerebral innate immunity. Trends Immunol 28: 138–145.
Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E (2005). Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol 159: 12–19.
Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X et al (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry 21: 1358–1365.
Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP (2014). Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry 76: 575–584.
Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D et al (2016). Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351: aac9698 This is a larger study combining several smaller studies to propse a mechanism by which medial prefrontal cortex modulates expression of reward-seeking behavior.
Figiel I, Dzwonek K (2007). TNFalpha and TNF receptor 1 expression in the mixed neuronal-glial cultures of hippocampal dentate gyrus exposed to glutamate or trimethyltin. Brain Res 1131: 17–28.
Figiel M, Allritz C, Lehmann C, Engele J (2007). Gap junctional control of glial glutamate transporter expression. Mol Cell Neurosci 35: 130–137.
Figuera-Losada M, Rojas C, Slusher BS (2014). Inhibition of microglia activation as a phenotypic assay in early drug discovery. J Biomol Screen 19: 17–31.
Fischer R, Wajant H, Kontermann R, Pfizenmaier K, Maier O (2014). Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia 62: 272–283.
Fontana A, Fierz W, Wekerle H (1984). Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307: 273–276.
Friedman H, Klein T, Specter S, Newton C, Nowotny A (1990). Immunoadjuvanticity of endotoxins and nontoxic derivatives for normal and leukemic immunocytes. Adv Exp Med Biol 256: 525–535.
Gamboa C, Ortega A (2002). Insulin-like growth factor-1 increases activity and surface levels of the GLAST subtype of glutamate transporter. Neurochem Int 40: 397–403.
Ganor Y, Besser M, Ben-Zakay N, Unger T, Levite M (2003). Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin-mediated adhesion to laminin and fibronectin and chemotactic migration. J Immunol 170: 4362–4372 This study provided the first account doumenting presence AMPA receptors in T-cells and suggested that these receptors might be involved in autoantbodies to autoreceptor encephalopathies.
Garg SK, Banerjee R, Kipnis J (2008). Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol 180: 3866–3873.
Garg SK, Kipnis J, Banerjee R (2009). IFN-gamma and IL-4 differentially shape metabolic responses and neuroprotective phenotype of astrocytes. J Neurochem 108: 1155–1166.
Gautier HO, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I et al (2015). Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat Commun 6: 8518 The paper suggests that neuronal activity and release of glutamate instruct oligodendrocyte precursors to differentiate into newer myelinating oligodendrocytes to promote repair. This study has far reaching implications into our understanding of the process of neural repair and neural plasticity.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S et al (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845.
Giovanoli S, Weber-Stadlbauer U, Schedlowski M, Meyer U, Engler H (2016). Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Behav Immun 55: 25–38.
Godbout JP, Johnson RW (2009). Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin North Am 29: 321–337.
Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O’Connor J et al (2008). Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology 33: 2341–2351.
Goldsmith DR, Rapaport MH, Miller BJ (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry (e-pub ahead of print; doi:10.1038/mp.2016.3).
Gourley SL, Espitia JW, Sanacora G, Taylor JR (2012). Antidepressant-like properties of oral riluzole and utility of incentive disengagement models of depression in mice. Psychopharmacology 219: 805–814.
Graeber MB, Streit WJ (2010). Microglia: biology and pathology. Acta Neuropathol 119: 89–105.
Gras G, Samah B, Hubert A, Leone C, Porcheray F, Rimaniol AC (2012). EAAT expression by macrophages and microglia: still more questions than answers. Amino Acids 42: 221–229.
Guidetti P, Schwarcz R (1999). 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur J Neurosci 11: 3857–3863.
Guillemin GJ (2012). Quinolinic acid, the inescapable neurotoxin. FEBS J 279: 1356–1365 This paper reviews some of the most recent findings about the effects of QUIN as a neurotoxin, gliotoxin, proinflammatory mediator, pro-oxidant molecule that can alter synaptic integrity.
Guyon A (2014). CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci 8: 65.
Hamidi M, Drevets WC, Price JL (2004). Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry 55: 563–569.
Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I et al (2013). The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain Behav Immun 33: 131–138.
Hardingham GE (2009). Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem Soc Trans 37 (Pt 6): 1147–1160.
Hardingham GE, Bading H (2010). Synaptic vs extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11: 682–696 This is a pioneering work on how perturbations in the balance between synaptic and extrasynaptic NMDAR activity contribute to neuronal dysfunction in short term and neuropathology in the long term.
Hardingham GE, Fukunaga Y, Bading H (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5: 405–414.
Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR et al (2015). Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: preliminary findings. Brain Behav Immun 46: 17–22.
Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T et al (2016). Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry 21: 1351–1357 This is the first paper linking increased inflammation to increased glutamate and glial dysfunction in the basal ganglia regions of patients with major depression.
Haroon E, Raison CL, Miller AH (2012). Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37: 137–162 A previous Neuropsychopharmacology Review (NPPR) article providing a comprehensive review of the mechanisms by which cytokines influence behavior and might serve as a target of opportunity to treat mood disorders.
Haroon E, Watari K, Thomas A, Ajilore O, Mintz J, Elderkin-Thompson V et al (2009). Prefrontal myo-inositol concentration and visuospatial functioning among diabetic depressed patients. Psychiatry Res 171: 10–19.
Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR et al (2014). IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 39: 1777–1785 Using a longitudinal design, this paper documented that immune stimulation resulting from IFN-alpha administration in humans induced glutamate increases in anterior cingulate and basal ganglia regions associated with depressive symptoms.
Hashimoto K (2015). Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci 16: 7796–7801.
Haydon PG, Carmignoto G (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86: 1009–1031.
Heinisch S, Kirby LG (2010). SDF-1alpha/CXCL12 enhances GABA and glutamate synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuropharmacology 58: 501–514.
Helms HC, Madelung R, Waagepetersen HS, Nielsen CU, Brodin B (2012). In vitro evidence for the brain glutamate efflux hypothesis: brain endothelial cells cocultured with astrocytes display a polarized brain-to-blood transport of glutamate. Glia 60: 882–893.
Howren MB, Lamkin DM, Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71: 171–186.
Hsuchou H, Pan W, Barnes MJ, Kastin AJ (2009). Leptin receptor mRNA in rat brain astrocytes. Peptides 30: 2275–2280.
Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC (2000). Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 7: 153–159.
Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H (2008). Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett 432: 232–236.
Iliff JJ, Nedergaard M (2013). Is there a cerebral lymphatic system? Stroke 44 (6 Suppl 1): S93–S95.
Innocenti B, Parpura V, Haydon PG (2000). Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci 20: 1800–1808.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K et al (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167: 748–751.
Iori V, Frigerio F, Vezzani A (2016). Modulation of neuronal excitability by immune mediators in epilepsy. Curr Opin Pharmacol 26: 118–123.
Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL et al (2006). Astrocytes promote myelination in response to electrical impulses. Neuron 49: 823–832.
Iwata M, Ota KT, Duman RS (2013). The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 31: 105–114.
Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S et al (2016). Psychological stress activates the inflammasome via release of Adenosine Triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry 80: 12–22 This is a ground-breaking paper demonstrating that psychological ‘stress’ is sensed by the innate immune system in the microglia via NLRP3 inflammasome cascade.
Jackman NA, Uliasz TF, Hewett JA, Hewett SJ (2010). Regulation of system x(c)(-)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia 58: 1806–1815.
Jaso BA, Niciu MJ, Iadarola ND, Lally N, Richards EM, Park M et al (2016). Therapeutic modulation of glutamate receptors in major depressive disorder. Curr Neuropharmacol. (e-pub ahead of print; pii:CN-EPUB-74491).
Jebelli J, Su W, Hopkins S, Pocock J, Garden GA (2015). Glia: guardians, gluttons, or guides for the maintenance of neuronal connectivity? Ann NY Acad Sci 1351: 1–10.
Johnstone M, Gearing AJ, Miller KM (1999). A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol 93: 182–193.
Kalandadze A, Wu Y, Fournier K, Robinson MB (2004). Identification of motifs involved in endoplasmic reticulum retention-forward trafficking of the GLT-1 subtype of glutamate transporter. J Neurosci 24: 5183–5192.
Karpuk N, Burkovetskaya M, Kielian T (2012). Neuroinflammation alters voltage-dependent conductance in striatal astrocytes. J Neurophysiol 108: 112–123.
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011). Physiology of microglia. Physiol Rev 91: 461–553 This paper masterfully synthesizes how microglial cells are the best sensors of brain pathology due to their dual functions on immune and neural systems.
Kettenmann H, Ransom BR (2012) Neuroglia. Oxford University Press: New York, NY An authoritative textbook and master reference on all aspects of glial functioning.
Khakh BS, Sofroniew MV (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18: 942–952 This is a comprehensive review that heralds the concept that astrocytes play central role in health and pathology in a context-dependent manner.
Kielian T, Bearden ED, Baldwin AC, Esen N (2004). IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol 63: 381–396.
Kielian T, Esen N (2004). Effects of neuroinflammation on glia-glia gap junctional intercellular communication: a perspective. Neurochem Int 45: 429–436.
Kigerl KA, Ankeny DP, Garg SK, Wei P, Guan Z, Lai W et al (2012). System x(c)(-) regulates microglia and macrophage glutamate excitotoxicity in vivo. Exp Neurol 233: 333–341.
Kim H, McGrath BM, Silverstone PH (2005). A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders—focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol 20: 309–326.
Kimelberg HK, Nedergaard M (2010). Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7: 338–353.
Kipnis J, Gadani S, Derecki NC (2012). Pro-cognitive properties of T cells. Nat Rev Immunol 12: 663–669.
Korn T, Magnus T, Jung S (2005). Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB J 19: 1878–1880.
Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34: 1397–1411.
Le Y, Zhou Y, Iribarren P, Wang J (2004). Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol 1: 95–104.
Leibowitz A, Boyko M, Shapira Y, Zlotnik A (2012). Blood glutamate scavenging: insight into neuroprotection. Int J Mol Sci 13: 10041–10066.
Levite M (2014). Glutamate receptor antibodies in neurological diseases. J Neural Transm (Vienna) 121: 1029–1075.
Levy LM, Warr O, Attwell D (1998). Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci 18: 9620–9628.
Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW et al (2013). The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18: 522–555 This is an authoritative reference that summarizes the current knowledge regarding the molecular mechanisms regulating xC- systems.
Lewerenz J, Maher P (2015). Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front Neurosci 9: 469.
Li X, Wallin C, Weber SG, Sandberg M (1999). Net efflux of cysteine, glutathione and related metabolites from rat hippocampal slices during oxygen/glucose deprivation: dependence on gamma-glutamyl transpeptidase. Brain Res 815: 81–88.
Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW (2004). Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem 89: 1092–1100.
Liddelow S, Barres B (2015). SnapShot: astrocytes in health and disease. Cell 162: 1170–1170 e1171.
Longuemare MC, Swanson RA (1995). Excitatory amino acid release from astrocytes during energy failure by reversal of sodium-dependent uptake. J Neurosci Res 40: 379–386.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD et al (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341.
Ma TM, Paul BD, Fu C, Hu S, Zhu H, Blackshaw S et al (2014). Serine racemase regulated by binding to stargazin and PSD-95: potential N-methyl-D-aspartate-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (NMDA-AMPA) glutamate neurotransmission cross-talk. J Biol Chem 289: 29631–29641 This recently published paper provides breakthrough information on an entirely novel mode of synaptic regulation by the enzyme serine racemase in promoting the cross-talk between NMDA and AMPA receptors.
Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID et al (2016). The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry in press). This is a recent paper suggesting that adipokines may either predict response or have a role in the possible therapeutic effects of ketamine.
Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA Jr (2009). Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther 123: 143–150.
Malarkey EB, Parpura V (2008). Mechanisms of glutamate release from astrocytes. Neurochem Int 52: 142–154 This is a comprehensive synthesis of mechanisms by which immune factors promote glutamate release.
Marz P, Heese K, Dimitriades-Schmutz B, Rose-John S, Otten U (1999). Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia 26: 191–200.
Massie A, Boillee S, Hewett S, Knackstedt L, Lewerenz J (2015). Main path and byways: non-vesicular glutamate release by system xc(-) as an important modifier of glutamatergic neurotransmission. J Neurochem 135: 1062–1079.
Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S et al (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science. (e-pub ahead of print; doi:10.1126/science.aad8670).
Mateo C, Avermann M, Gentet LJ, Zhang F, Deisseroth K, Petersen CC (2011). In vivo optogenetic stimulation of neocortical excitatory neurons drives brain-state-dependent inhibition. Curr Biol 21: 1593–1602.
Mathew S, Fava M, Guerguieva R, Sanacora G (eds) (2015). A Randomized Placebo-Controlled Adjunctive Trial of Riluzole in Treatment-Resistant Major Depressive Disorder—NCT01204918—Efficacy and Tolerability of Riluzole in Treatment Resistant Depression. ASCP Annual Meeting (NCDEU). Miami, FL, USA.
Matute C (2006). Oligodendrocyte NMDA receptors: a novel therapeutic target. Trends Mol Med 12: 289–292.
Matute C, Domercq M, Sanchez-Gomez MV (2006). Glutamate-mediated glial injury: mechanisms and clinical importance. Glia 53: 212–224.
McCullumsmith RE, Sanacora G (2015). Regulation of extrasynaptic glutamate levels as a pathophysiological mechanism in disorders of motivation and addiction. Neuropsychopharmacology 40: 254–255 This brief paper summarizes evidence that diminished perisynaptic glutamate buffering and reuptake by EAATs might be a common pathophysiological mechanism in psychiatric illness.
McEwen BS, Nasca C, Gray JD (2016). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41: 3–23.
Medina A, Watson SJ, Bunney W Jr, Myers RM, Schatzberg A, Barchas J et al (2016). Evidence for alterations of the glial syncytial function in major depressive disorder. J Psychiatr Res 72: 15–21.
Merzenich MM, Van Vleet TM, Nahum M (2014). Brain plasticity-based therapeutics. Front Hum Neurosci 8: 385.
Mesci P, Zaidi S, Lobsiger CS, Millecamps S, Escartin C, Seilhean D et al (2015). System xC- is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice. Brain 138 (Pt 1): 53–68.
Miguel-Hidalgo JJ, Wilson BA, Hussain S, Meshram A, Rajkowska G, Stockmeier CA (2014). Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. J Psychiatr Res 55: 101–109.
Miller AH, Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16: 22–34 A comprehensive review of most updated concepts in immune mediation of depressive and mood disorders and ties it to our evolutionary origins. An excellent, thoughtful introduction to the field of behavioral immunology.
Miller KR, Streit WJ (2007). The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol 3: 245–253.
Milton ID, Banner SJ, Ince PG, Piggott NH, Fray AE, Thatcher N et al (1997). Expression of the glial glutamate transporter EAAT2 in the human CNS: an immunohistochemical study. Brain Res Mol Brain Res 52: 17–31.
Mineur YS, Picciotto MR, Sanacora G (2007). Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol Psychiatry 61: 250–252.
Mizuno T, Zhang G, Takeuchi H, Kawanokuchi J, Wang J, Sonobe Y et al (2008). Interferon-gamma directly induces neurotoxicity through a neuron specific, calcium-permeable complex of IFN-gamma receptor and AMPA GluR1 receptor. FASEB J 22: 1797–1806.
Moylan S, Maes M, Wray NR, Berk M (2013). The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18: 595–606 A well-written synthesis summarizing the basic, clinical and neuroimaging datat supporting concept of neuroprogression in mood disorders.
Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B (2007). Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 98: 143–151.
Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C et al (2015). Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry 20: 320–328.
Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O (2013). Neuroinflammation and psychiatric illness. J Neuroinflammation 10: 43.
Nakagawa T, Otsubo Y, Yatani Y, Shirakawa H, Kaneko S (2008). Mechanisms of substrate transport-induced clustering of a glial glutamate transporter GLT-1 in astroglial-neuronal cultures. Eur J Neurosci 28: 1719–1730.
Nathan C, Cunningham-Bussel A (2013). Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol 13: 349–361 This is a specialized review on the role played by immune factors in promoting oxidative stress.
Nedergaard M, Takano T, Hansen AJ (2002). Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci 3: 748–755 A classic review of the current knowledge on glutamatergic signalling in non-neuronal cells, with a special emphasis on astrocytes.
Niciu MJ, Ionescu DF, Richards EM, Zarate CA Jr (2014a). Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm 121: 907–924 This paper reviews evidence implicating glutamatergic dysfunction in MDD and supports the use of glutamate receptor modulators for treatment-resistant depression.
Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED et al (2014b). Riluzole likely lacks antidepressant efficacy in ketamine non-responders. J Psychiatr Res 58: 197–199.
Nimmerjahn A, Kirchhoff F, Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318 By usingin vivo two-photon imaging, this was the first report to demonstrate that microglial cells are highly active even during their presumed resting state.
Noda M, Nakanishi H, Nabekura J, Akaike N (2000). AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci 20: 251–258.
Norden DM, Godbout JP (2013). Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39: 19–34.
Norden DM, Muccigrosso MM, Godbout JP (2015a). Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96 (Pt A): 29–41.
Norden DM, Trojanowski PJ, Walker FR, Godbout JP (2016). Insensitivity of astrocytes to interleukin 10 signaling following peripheral immune challenge results in prolonged microglial activation in the aged brain. Neurobiol Aging 44: 22–41.
Norden DM, Trojanowski PT, Walker FR, Godbout JP (2015b). Age-related impairments in the dynamic regulation of active microglia by astrocytes. Brain Behav Immun 49: e16–e17.
Ohgoh M, Hanada T, Smith T, Hashimoto T, Ueno M, Yamanishi Y et al (2002). Altered expression of glutamate transporters in experimental autoimmune encephalomyelitis. J Neuroimmunol 125: 170–178.
Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ (1993). Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci 13: 1441–1453.
Olmos G, Llado J (2014). Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014: 861231.
Ongur D, Bechtholt AJ, Carlezon WA Jr, Cohen BM (2014). Glial abnormalities in mood disorders. Harv Rev Psychiatry 22: 334–337.
Ongur D, Drevets WC, Price JL (1998). Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 95: 13290–13295 The first histological study to report that numbers of glia were reduced markedly in both MDD and BD.
Oxenkrug G (2011). Interferon-gamma—inducible inflammation: contribution to aging and aging-associated psychiatric disorders. Aging Dis 2: 474–486.
Oxenkrug GF (2010). Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann NY Acad Sci 1199: 1–14.
Pan W, Hsuchou H, Jayaram B, Khan RS, Huang EY, Wu X et al (2012). Leptin action on nonneuronal cells in the CNS: potential clinical applications. Ann NY Acad Sci 1264: 64–71.
Parpura V, Verkhratsky A (2013). Astroglial amino acid-based transmitter receptors. Amino Acids 44: 1151–1158.
Parri HR, Gould TM, Crunelli V (2001). Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4: 803–812.
Persson M, Brantefjord M, Hansson E, Ronnback L (2005). Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia 51: 111–120.
Petrelli F, Bezzi P (2016). Novel insights into gliotransmitters. Curr Opin Pharmacol 26: 138–145 A masteful synthesis of two decades of work demonstrating that astrocytes play an active role in neuron communication by releasing neuro-active gliotransmitters into extra-cellular spaces.
Piani D, Fontana A (1994). Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol 152: 3578–3585.
Piani D, Frei K, Do KQ, Cuenod M, Fontana A (1991). Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett 133: 159–162.
Piers TM, Heales SJ, Pocock JM (2011). Positive allosteric modulation of metabotropic glutamate receptor 5 down-regulates fibrinogen-activated microglia providing neuronal protection. Neurosci Lett 505: 140–145.
Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G (2008). Riluzole in the treatment of mood and anxiety disorders. CNS Drugs 22: 761–786.
Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gomez-Cruz G, Leon-Ortiz P et al (2016). Elevated myo-inositol, choline, and glutamate levels in the associative striatum of antipsychotic-naive patients with first-episode psychosis: a proton magnetic resonance spectroscopy study with implications for glial dysfunction. Schizophr Bull 42: 415–424.
Pocock JM, Kettenmann H (2007). Neurotransmitter receptors on microglia. Trends Neurosci 30: 527–535.
Popoli M, Yan Z, McEwen BS, Sanacora G (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13: 22–37 This ia comprehensive review of the effects of stress and glucocorticoids on glutamate release, glutamate receptors and glutamate clearance and metabolism.
Pribiag H, Stellwagen D (2014). Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology 78: 13–22 This is a recent review summarizing evidence implicating molecules such as pro-inflammatory cytokine TNFalpha in the modulation of synaptic and hebbian plasticity.
Pribiag M, Stellwagen D (2013) Neuroimmune modulation of synaptic function In: Cui C, Grandison L, Noronha A(eds) Neural-Immune Interations in Brain Functions and Alcoho-Related Disorders. Springer: New York, NY, USA. pp 65–94.
Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G et al (2010). CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15: 393–403.
Rajkowska G, Mahajan G, Maciag D, Sathyanesan M, Iyo AH, Moulana M et al (2015). Oligodendrocyte morphometry and expression of myelin—Related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res 65: 53–62.
Rajkowska G, Miguel-Hidalgo JJ (2007). Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets 6: 219–233.
Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY et al (1999). Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45: 1085–1098.
Rajkowska G, Stockmeier CA (2013). Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14: 1225–1236.
Ransohoff RM (2016). A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19: 987–991 A perspective/review on the complexity of classifying microglial activation states.
Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM et al (2007). Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci 27: 6607–6619.
Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T et al (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300: 141–154.
Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O (1999). Reference distributions for the negative acute-phase proteins, albumin, transferrin, and transthyretin: a comparison of a large cohort to the world’s literature. J Clin Lab Anal 13: 280–286.
Rossi D (2015). Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol 130: 86–120 This review promotes astrocytes as intelligent ‘hubs’ maintaining neural connectivity and circuit plasticity.
Rossi DJ, Oshima T, Attwell D (2000). Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403: 316–321.
Rothstein JD (1996). Excitotoxicity hypothesis. Neurology 47 (4 Suppl 2): S19–S25.
Salardini E, Zeinoddini A, Mohammadinejad P, Khodaie-Ardakani MR, Zahraei N, Zeinoddini A et al (2016). Riluzole combination therapy for moderate-to-severe major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Psychiatr Res 75: 24–30.
Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H (2011). Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34: 3–11.
Sanacora G, Banasr M (2013). From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73: 1172–1179 This is a comprehensive synthesis of glial dysfunction in the pathophysiology of major depressive disorder.
Sanacora G, Treccani G, Popoli M (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62: 63–77.
Sandiego CM, Gallezot JD, Pittman B, Nabulsi N, Lim K, Lin SF et al (2015). Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci U S A 112: 12468–12473.
Santello M, Bezzi P, Volterra A (2011). TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69: 988–1001.
Santello M, Volterra A (2012). TNFalpha in synaptic function: switching gears. Trends Neurosci 35: 638–647.
Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS et al (2015). Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40: 463–471.
Schafer DP, Stevens B (2015). Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol 7: a020545.
Schluter K, Figiel M, Rozyczka J, Engele J (2002). CNS region-specific regulation of glial glutamate transporter expression. Eur J Neurosci 16: 836–842.
Schwarcz R (2004). The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 4: 12–17.
Schwarcz R (2016). Kynurenines and glutamate: multiple links and therapeutic implications. Adv Pharmacol 76: 13–37 This paper offers a masteful synthesis of how kynurenine pathway metabolties influence glutamatergic activity through direct effects on ionotropic and metabotropic glutamate receptors, vesicular glutamate transport and indirect effects at other recognition sites.
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ (2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13: 465–477 This paper explains the mechanims underlying the dysregulation of the kynurenine pathway resulting in the release of hyper-or hypofunction of active metabolites are associated with psychiatric diseases such as depression and schizophrenia.
Schwarcz R, Pellicciari R (2002). Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 303: 1–10.
Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V et al (2009). Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One 4: e6585.
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G et al (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72: 268–275.
Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE (2013). Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr Res 144: 1–8.
Sheng M, Hoogenraad CC (2007). The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem 76: 823–847.
Shrikant P, Benveniste EN (1996). The central nervous system as an immunocompetent organ: role of glial cells in antigen presentation. J Immunol 157: 1819–1822.
Sofroniew MV, Vinters HV (2010). Astrocytes: biology and pathology. Acta Neuropathol 119: 7–35.
Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, Zamvil SS (1998). Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol 161: 5959–5966.
Spranger M, Lindholm D, Bandtlow C, Heumann R, Gnahn H, Naher-Noe M et al (1990). Regulation of nerve growth factor (NGF) synthesis in the rat central nervous system: comparison between the effects of interleukin-1 and various growth factors in astrocyte cultures and in vivo. Eur J Neurosci 2: 69–76.
Stone TW, Forrest CM, Darlington LG (2012). Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J 279: 1386–1397.
Streit WJ, Xue QS (2013). Microglial senescence. CNS Neurol Disord Drug Targets 12: 763–767 This is an important review focussing on the failure of microglial functioning as seen in senile dementia.
Sun JD, Liu Y, Yuan YH, Li J, Chen NH (2012). Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 37: 1305–1320.
Szymocha R, Akaoka H, Dutuit M, Malcus C, Didier-Bazes M, Belin MF et al (2000). Human T-cell lymphotropic virus type 1-infected T lymphocytes impair catabolism and uptake of glutamate by astrocytes via Tax-1 and tumor necrosis factor alpha. J Virol 74: 6433–6441.
Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW (2003a). Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol 53: 588–595.
Takahashi K, Foster JB, Lin CL (2015). Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci 72: 3489–3506.
Takahashi T, Svoboda K, Malinow R (2003b). Experience strengthening transmission by driving AMPA receptors into synapses. Science 299: 1585–1588.
Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y, Sato K (2012). L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J Neuroinflammation 9: 275.
Takeuchi H, Jin S, Suzuki H, Doi Y, Liang J, Kawanokuchi J et al (2008). Blockade of microglial glutamate release protects against ischemic brain injury. Exp Neurol 214: 144–146.
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R et al (2006). Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281: 21362–21368.
Takeuchi H, Suzumura A (2014). Gap junctions and hemichannels composed of connexins: potential therapeutic targets for neurodegenerative diseases. Front Cell Neurosci 8: 189.
Tavares RG, Tasca CI, Santos CE, Alves LB, Porciuncula LO, Emanuelli T et al (2002). Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int 40: 621–627.
Taylor DL, Jones F, Kubota ES, Pocock JM (2005). Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci 25: 2952–2964.
Tilleux S, Hermans E (2007). Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 85: 2059–2070 This comprehensive review summarizes data demonstrating the impact of inflammatory mediators and related free radicals on the expression and activity of glial glutamate transporters.
Torres-Platas SG, Comeau S, Rachalski A, Bo GD, Cruceanu C, Turecki G et al (2014a). Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation 11: 12.
Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N (2014b). Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42: 50–59.
Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonte B, Turecki G et al (2011). Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology 36: 2650–2658.
Town T, Nikolic V, Tan J (2005). The microglial ‘activation’ continuum: from innate to adaptive responses. J Neuroinflammation 2: 24.
Tzingounis AV, Wadiche JI (2007). Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci 8: 935–947 This review describes glutamate buffering—a process by which glutamate transporters influence synaptic transmission and regulate synaptic plasticity.
Valkanova V, Ebmeier KP, Allan CL (2013). CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 150: 736–744.
Vecsei L, Szalardy L, Fulop F, Toldi J (2013). Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 12: 64–82.
Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL et al (2007). Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J Neuropathol Exp Neurol 66: 732–739.
Verkharatsky A, Butt A (2013) Glial Physiology and Pathophysiology. Wiley-Blackwell: West Sussex, UK A masterful compendium of all details pertaining to glial function and dysfunction.
Verkhratsky A, Nedergaard M, Hertz L (2016). Why are astrocytes important? Neurochem Res 40: 389–401 A thorough exposition of "astroglial cradles" and their role in the genesis, maturation and maintenance of synaptic connectivity.
Verkhratsky A, Rodriguez JJ, Parpura V (2014). Neuroglia in ageing and disease. Cell Tissue Res 357: 493–503.
Verkhratsky A, Steardo L, Parpura V, Montana V (2016). Translational potential of astrocytes in brain disorders. Prog Neurobiol 144: 188–205.
Vezzani A, Viviani B (2015). Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96 (Pt A): 70–82.
Viviani B, Boraso M (2011). Cytokines and neuronal channels: a molecular basis for age-related decline of neuronal function? Exp Gerontol 46: 199–206.
Volterra A, Meldolesi J (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6: 626–640.
Walker AJ, Foley BM, Sutor SL, McGillivray JA, Frye MA, Tye SJ (2015). Peripheral proinflammatory markers associated with ketamine response in a preclinical model of antidepressant-resistance. Behav Brain Res 293: 198–202.
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B et al (2013). NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38: 1609–1616 This is an important paper with findings that indicate that LPS-induced depressive-like behavior is mediated by NMDA receptor activation.
Walsh JG, Muruve DA, Power C (2014a). Inflammasomes in the CNS. Nat Rev Neurosci 15: 84–97.
Walsh JT, Watson N, Kipnis J (2014b). T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology 141: 340–344.
Wang F, Smith NA, Xu Q, Goldman S, Peng W, Huang JH et al (2013a). Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci 33: 17404–17412.
Wang ZC, Zhao J, Li S (2013b). Dysregulation of synaptic and extrasynaptic N-methyl-D-aspartate receptors induced by amyloid-beta. Neurosci Bull 29: 752–760.
Wei L, Simen A, Mane S, Kaffman A (2012). Early life stress inhibits expression of a novel innate immune pathway in the developing hippocampus. Neuropsychopharmacology 37: 567–580.
Wei R, Jonakait GM (1999). Neurotrophins and the anti-inflammatory agents interleukin-4 (IL-4), IL-10, IL-11 and transforming growth factor-beta1 (TGF-beta1) down-regulate T cell costimulatory molecules B7 and CD40 on cultured rat microglia. J Neuroimmunol 95: 8–18.
Whitton AE, Treadway MT, Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry 28: 7–12.
Wohleb ES, Franklin T, Iwata M, Duman RS (2016). Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 17: 497–511.
Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF et al (2014). Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry 75: 970–981 This paper reported that repeated social defeat induced macrophage recruitment into the brain corresponded with development and persistence of anxiety-like behavior.
Woodbury-Farina MA (2014). The importance of glia in dealing with stress. Psychiatr Clin North Am 37: 679–705.
Xie Z, Morgan TE, Rozovsky I, Finch CE (2003). Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol 182: 135–141.
Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang Q et al (2016). Systemic inflammation induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav Immun 56: 352–362.
Ye ZC, Sontheimer H (1996). Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport 7: 2181–2185.
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG et al (2012). Genomic analysis of reactive astrogliosis. J Neurosci 32: 6391–6410.
Zarate C Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G (2010). Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry 18: 293–303.
Zeevalk GD, Bernard LP, Sinha C, Ehrhart J, Nicklas WJ (1998). Excitotoxicity and oxidative stress during inhibition of energy metabolism. Dev Neurosci 20: 444–453.
Zerangue N, Kavanaugh MP (1996). Flux coupling in a neuronal glutamate transporter. Nature 383: 634–637.
Zhou Y, Danbolt NC (2014). Glutamate as a neurotransmitter in the healthy brain. J Neural Transm 121: 799–817 This is a masteful sythesis of neurophysiology of glutamate.
Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA (2010). Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology 58: 465–473.
Zou JY, Crews FT (2005). TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res 1034: 11–24 A pioneering paper reporting that TNF alpha potentiates glutamate neurotoxicity by blocking glutamate transporter activity.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Haroon, E., Miller, A. & Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacol 42, 193–215 (2017). https://doi.org/10.1038/npp.2016.199
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.199
This article is cited by
-
MM165 - A Small Hybrid Molecule Modulates the Kynurenine Pathway and Attenuates Lipopolysaccharide-Induced Memory Deficits and Inflammation
Neurochemical Research (2024)
-
Drug repositioning targeting glutaminase reveals drug candidates for the treatment of Alzheimer’s disease patients
Journal of Translational Medicine (2023)
-
α7 Nicotinic acetylcholine receptor: a key receptor in the cholinergic anti-inflammatory pathway exerting an antidepressant effect
Journal of Neuroinflammation (2023)
-
The connexin hemichannel inhibitor D4 produces rapid antidepressant-like effects in mice
Journal of Neuroinflammation (2023)
-
Neuroprotective compounds from marine invertebrates
Beni-Suef University Journal of Basic and Applied Sciences (2023)