Abstract
Depression and metabolic disorders, including overweight and obesity, appear tightly interrelated. The prevalence of these conditions is concurrently growing worldwide, and both depression and overweight/obesity represent substantial risk factors for multiple medical complications. Moreover, there is now multiple evidence for a bidirectional relationship between depression and increased adiposity, with overweight/obesity being associated with an increased prevalence of depression, and in turn, depression augmenting the risk of weight gain and obesity. Although the reasons for this intricate link between depression and increased adiposity remain unclear, converging clinical and preclinical evidence points to a critical role for inflammatory processes and related alterations of brain functions. In support of this notion, increased adiposity leads to a chronic low-grade activation of inflammatory processes, which have been shown elsewhere to have a potent role in the pathophysiology of depression. It is therefore highly possible that adiposity-driven inflammation contributes to the development of depressive disorders and their growing prevalence worldwide. This review will present recent evidence in support of this hypothesis and will discuss the underlying mechanisms and potential therapeutic targets. Altogether, findings presented here should help to better understand the mechanisms linking adiposity to depression and facilitate the identification of new preventive and/or therapeutic strategies.
Similar content being viewed by others
INTRODUCTION
Mental disorders account for almost 20% of the burden of disease worldwide, and they affect approximately 25% of the population during lifetime. Based on the Global Burden of Disease Study, depression is the most common of those disorders, representing the leading chronic condition and the first cause of years lived with disability (Whiteford et al, 2013). This disorder relates to a substantial increased risk of morbidity and death and is associated with a significant societal and economic burden. Although advances have been made in the treatment of depression, its prevalence is continuing to rise, notably owing to stagnation in the development of pharmacological treatments, the growth and aging of the population, and the increasing prevalence of chronic medical conditions, such as metabolic disorders and obesity, which are associated with an increased risk of depressive comorbidity. This review will discuss the relevance of increased adiposity to the development of depression and the role of inflammation in promoting this effect.
FAT AND DEPRESSION: A VICIOUS CIRCLE
Obesity and depression currently represent substantial public health concerns, as the prevalence of these two conditions is growing worldwide. Albeit distinguishable in terms of etiopathological processes, mounting evidence suggests intricate bidirectional relationships between adiposity and depression (Luppino et al, 2010), which may explain their similar and parallel growth. Although depression relates to an increased risk of weight gain and obesity, overweight and obesity are in turn associated with a higher vulnerability for depressive disorders.
Economical changes together with modifications of lifestyle and diet habits represent potent contributors to the growing incidence of overweight and obesity worldwide. Primarily owing to an energy imbalance between calories consumed and calories expended, with an excessive consumption of energy-dense (high-fat and high-sugar) foods together with a reduction of physical activity, the pandemic of obesity is associated with a substantial economic burden and increasing medical costs (Hammond and Levine, 2010). In support of this, overweight and obesity are not only frequently associated with comorbidities and medical complications, including metabolic, endocrine, and cardiovascular diseases (CVD), but also neuropsychiatric disorders, such as depression (Dawes et al, 2016; Duarte-Guerra et al, 2015; Evans et al, 2005; Mather et al, 2009; Petry et al, 2008).
The incidence of depression in obese individuals is close to 30% (Dawes et al, 2016; Evans et al, 2005; Lasselin and Capuron, 2014a; Simon et al, 2008), a rate that is significantly higher than the one measured in the general population. Consistent with this, being obese was found to be associated with a risk of developing depression ranging from 1.18 to 5.25 depending on studies and methods of assessment (de Wit et al, 2010; Kasen et al, 2008; Ma and Xiao, 2010; Simon et al, 2008) and to predict recurrence of depressive episodes as well as antidepressant non-response in depressed patients (Kloiber et al, 2007; Nigatu et al, 2015; Oskooilar et al, 2009; Rizvi et al, 2014; Woo et al, 2016). Conversely, and supporting the bidirectional relationship between obesity and depression, the rates of obesity are particularly high in depressed patients, and depression was found to represent a strong predictor of weight gain and obesity, probably owing to effects of antidepressant medications and changes in eating behaviors and lifestyle (Lasserre et al, 2014; Lee et al, 2016; Simon et al, 2008). Moreover, psychosocial stress, a notorious risk factor for depression, is also known to contribute to weight gain and metabolic alterations and to participate in the development of obesity (Chuang et al, 2010; Kendler et al, 1999; Sinha and Jastreboff, 2013).
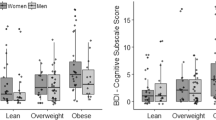
Usually greater in women (Carpenter et al, 2000; de Wit et al, 2010; Heo et al, 2006; Mitchell et al, 2012), depressive symptoms that develop in obese individuals often present atypical features such as increased appetite/weight gain, mood reactivity, and hypersomnia/fatigue (Chou and Yu, 2013; Levitan et al, 2012; Lojko et al, 2015). In agreement with this, overweight, obesity, and metabolic abnormalities, including leptin deregulation, appear to be more prevalent in atypical depression than in melancholic/typical depression (Chou and Yu, 2013; Cizza et al, 2012; Lamers et al, 2010; Lasserre et al, 2014; Levitan et al, 2012; Milaneschi et al, 2015). Interestingly, the association of atypical depression with leptin alterations was found to be stronger for increased adiposity levels (Milaneschi et al, 2015), pointing to a role for adiposity in this relationship. This finding also supports the hypothesis that depression with atypical features represents a metabolic subtype of depression likely related to adiposity (Lamers et al, 2010; Penninx et al, 2013). Moreover, and strengthening the notion of a bidirectional relationship between depression and obesity, atypical depression (notably through its association with increased appetite) is notorious for representing a significant risk factor for weight gain and obesity (Hasler et al, 2004).
In addition to atypical depression, bipolar depression was also found to be associated with adiposity and metabolic variations (Alciati et al, 2007; Grothe et al, 2014; Vannucchi et al, 2014). Patients with bipolar disorders exhibit higher rates of obesity and metabolic comorbidities, which are believed to significantly modulate disease outcomes (Crump et al, 2013; McIntyre et al, 2010). Consistent with this, it was recently found that adiponectin levels represent potent moderators of illness course in bipolar depression, suggesting the involvement of metabolic processes in the physiopathology of the illness (Mansur et al, 2016). Interestingly, and supporting further the notion of specific associations with atypical depressive features, obesity in women with established type I bipolar disorder relates to episodes of major depression with atypical characteristics (Pickering et al, 2007).
As described for chronic diseases, the occurrence of depression in patients with obesity and metabolic disorders is associated with various deleterious consequences. Not only it substantially impacts quality of life but it also compromises weight loss and compliance to treatment or weight management strategies (Brunault et al, 2012; Dixon et al, 2003; Evans et al, 2005; Herva et al, 2006; Kinzl et al, 2006; Mazzeschi et al, 2012). Importantly, depressive comorbidities in obesity facilitate the development of additional complications, including CVD and metabolic disorders. These complications may occur together or mutually facilitate their co-occurrence, leading to the instauration of a vicious circle promoting the development of multiple disorders in chain. In support of this, obesity and metabolic disorders represent potent risk factors for CVD that, in turn, are associated with an increased vulnerability for depressive disorders (Almas et al, 2015; Hare et al, 2014). Similarly, depression, notably when it occurs in a context of metabolic disorders, is known to represent a significant risk factor for the development of CVD (Martin et al, 2016; Wulsin and Singal, 2003). These coexisting complications suggest the involvement of common pathophysiological mechanisms among which inflammation represents a key candidate given its position at the interface between metabolic, vascular, and central nervous system pathways and its pivotal role in the etiology of disorders affecting these systems (Figure 1).
Inflammation as a link between adiposity, depression and related comorbidities. The relationship between adiposity and depression is bidirectional, with adiposity being associated with an increased prevalence of depression, and in turn, depression (in particular, with atypical features) augmenting the risk of overweight/obesity. Depressive comorbidities in obesity facilitate the development of additional complications, including cardiovascular diseases (CVD), metabolic syndrome and type 2 diabetes. Inflammation represents the underlying link between adiposity and depression and their effects on comorbidites, given its position at the interface between metabolic, vascular and central nervous system pathways and its pivotal role in the etiology of disorders affecting these different systems.
IS INFLAMMATION LINKING FAT TO DEPRESSION?
Inflammation: A Main Feature of Obesity
Along with metabolic deregulations, basal systemic low-grade inflammation and enhanced susceptibility to immune-mediated diseases appear as a key component of obesity (Kanneganti and Dixit, 2012), which is now considered as an inflammatory condition affecting both the innate and acquired immune systems (Cancello and Clement, 2006; Gregor and Hotamisligil, 2011; Schmidt and Duncan, 2003). Increased plasma levels of inflammatory cytokines (including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, and C-reactive protein (CRP)) and activation of inflammatory signaling pathways have been reported in both obese individuals (Capuron et al, 2011b; Park et al, 2005; Visser et al, 1999) and animal models of obesity (Cani et al, 2008; De Souza et al, 2005; Dinel et al, 2011, 2014; Lawrence et al, 2012; Pistell et al, 2010; Xu et al, 2003).
Different complementary mechanisms contribute to the progressive development of chronic low-grade inflammation associated with obesity. One of the main protagonists is the white adipose tissue, as shown by findings reporting associations between circulating levels of cytokines and measures of central adiposity (Park et al, 2005; Visser et al, 1999). Conversely, weight loss, induced either by low-caloric diet or bariatric surgery, significantly reduces peripheral inflammation in obese individuals (Belza et al, 2009; Hakeam et al, 2009; Manco et al, 2007; Rao, 2012) and animals (Liu et al, 2014; Schneck et al, 2014; Zhang et al, 2011). Adipocytes, together with infiltrated macrophages and T cells that progressively accumulate in the white adipose tissue, have indeed the ability to potently secrete inflammatory mediators (Cancello et al, 2006; Gregor and Hotamisligil, 2011; Kim et al, 2014; Lasselin et al, 2014b; Zeyda et al, 2011). Part of systemic inflammation in obesity also comes from other organs, in particular, the liver and muscles that are similarly infiltrated by activated immune cells (McNelis and Olefsky, 2014; Pedersen and Febbraio, 2012).
More recently, the gut microbiota has received increasing attention as an additional important player in the pathogenesis of obesity, particularly with regard to modulation of inflammation, energy metabolism, and body weight homeostasis (Cani et al, 2012; Cani et al, 2009; Finelli et al, 2014; Flint, 2011; Tehrani et al, 2012; Verdam et al, 2013). Although the gut microbiota consists of thousands of different bacterial species, two bacterial phyla corresponding, respectively, to the Gram-negative Bacteroidetes and the Gram-positive Firmicutes, are dominant in both humans and mice (Eckburg et al, 2005; Ley et al, 2005). Interestingly, obese individuals have lower bacterial diversity than lean subjects (Armougom et al, 2009; Turnbaugh et al, 2009) and impaired Bacteroidetes/Firmicutes ratio (Armougom et al, 2009; Cani et al, 2009; Ley et al, 2005; Verdam et al, 2013). Moreover, these alterations are related to markers of local, systemic, and brain inflammation and corrected after weight loss (Aron-Wisnewsky et al, 2012; Bruce-Keller et al, 2015; Cani et al, 2008; Furet et al, 2010). In overweight/obese humans, low fecal bacterial gene richness is also associated with higher overall adiposity and systemic inflammation (Cotillard et al, 2013; Le Chatelier et al, 2013). Abnormal increase in gut permeability to bacteria and their products, as reported in obesity, further contributes to the onset and progression of systemic inflammation (Brun et al, 2007). Chronic intake of high-fat diet in mice has been indeed shown to induce metabolic endotoxemia (ie, increased plasma levels of lipopolysaccharide (LPS)). By activating systemic macrophages through the binding of LPS to its Toll-like receptor-4, this endotoxemia increases in turn the inflammatory state in obesity (Cani et al, 2008; Cani et al, 2009; Verdam et al, 2013). Conversely, reduced serum levels of an endotoxemia marker, the LPS-binding protein, are found after weight loss in obese individuals (Cani et al, 2008; Yang et al, 2014).
Abundant evidence supports immune-to-brain communication, with peripheral cytokines acting on the brain to induce local production of cytokines (Anisman et al, 2008; Dantzer et al, 2008). Consistent with this, recent data highlight increased inflammatory processes in the brain of obese individuals (Buckman et al, 2013; Rummel et al, 2010). This is particularly notable in the hypothalamus, where clinical indication of glial activation has been reported (Thaler et al, 2012). Enhanced hypothalamic inflammatory cytokine expression and activation of dependent signaling pathways have also been repeatedly found in diet-induced obesity (DIO) models, both in baseline conditions or following an immune challenge (Andre et al, 2014; Cai and Liu, 2012; De Souza et al, 2005; Gao et al, 2014; Kleinridders et al, 2009; Maric et al, 2014; Pohl et al, 2009; Zhang et al, 2008). Central inflammation in obesity likely results from adiposity-related systemic inflammatory processes or endotoxemia induced by impaired gut permeability, as discussed above. This last assumption fits with mounting literature reporting the existence of a rich and complex communication network between the gut and the brain that involves endocrine, immune, and neural pathways (Grenham et al, 2011). Of note, the possibility that central inflammation may, on the contrary, represent an early event promoting the development of obesity following high-fat diet exposure cannot be excluded. Supporting this notion, blocking hypothalamic inflammation prevents high-fat diet-induced obesity and related metabolic alterations (Milanski et al, 2009; Zhang et al, 2008). In addition, prenatal cytokine exposure has been shown to promote the development of obesity at adulthood (Dahlgren et al, 2001). Whatever the case, it is now clear that increased hypothalamic inflammation is related to the metabolic deregulations that characterize severe obesity, including leptin resistance, insulin resistance, and hyperglycemia (De Souza et al, 2005; Kleinridders et al, 2009; Velloso et al, 2008; Zhang et al, 2008). By impairing hypothalamic peptidergic neuronal networks involved in the control of food intake and energy balance, inflammatory factors may also promote weight gain (Thaler and Schwartz, 2010; Velloso et al, 2008). Similar effects have been reported following chronic psychosocial stress, which induces both inflammation and metabolic alterations, including weight gain (Bierhaus et al, 2003; Chuang et al, 2010; Kleinridders et al, 2009). Interestingly, obesity-associated inflammation, notably as it relates to the visceral adipose tissue, was found to impact obesity treatment outcomes, with increased adipose expression of immune cells and inflammatory markers being associated with lower BMI reduction after bariatric surgery in severely obese patients (Lasselin et al, 2014b).
In addition to the hypothalamus, the hippocampus and cortex also display signs of neuroinflammation in rodent models of obesity (Dinel et al, 2011, 2014; Erion et al, 2014; Pistell et al, 2010). For example, increased systemic inflammation and/or reduced immune competence, which are reported in genetic models of severe obesity (ob/ob (deficient for leptin) and db/db (deficient for functional leptin receptor) mice), are associated with increased hippocampal cytokine expression (Dinel et al, 2011, 2014; Erion et al, 2014). Similarly, exacerbated hippocampal inflammation has been reported in DIO models (Andre et al, 2014; Boitard et al, 2014; Dinel et al, 2014). Consistent with these findings, clinical evidence shows an inverse association between activation of inflammatory processes and brain volume, notably in the hippocampus and prefrontal cortex (Meier et al, 2016; Savitz et al, 2014; Zunszain et al, 2012), and between brain volume and waist circumference and/or BMI (Janowitz et al, 2015; Pannacciulli et al, 2006; Yokum et al, 2012).
Neuropsychiatric Effects of Inflammation: Evidence and Mechanisms
Over the past decades, the key role of deregulated production and/or brain action of cytokines in the induction of neuropsychiatric disorders has been abundantly documented (Capuron and Miller, 2011a; Dantzer et al, 2008; Lasselin and Capuron, 2014a). Cytokines, released locally by activated innate immune cells in conditions of tissue injury, infection, or inflammation, are able to act systemically on distant organs, including the brain that they can reach through several non-exclusive humoral, neural, and cellular pathways, as reviewed elsewhere (Capuron and Miller, 2011a; Dantzer et al, 2008). Activation of immune-to-brain communication ultimately induces the production of brain cytokines by activated endothelial and glial cells, particularly microglia (Castanon et al, 2004; Laye et al, 1994; Ransohoff and Perry, 2009). Locally produced inflammatory cytokines activate the neuroendocrine system (in particular, the hypothalamic–pituitary–adrenal (HPA) axis), impair neurotransmitter metabolism and function, and alter neural plasticity and brain circuitry. These alterations, in turn, lead to a large number of behavioral changes (including weakness, listlessness, malaise, anorexia, fatigue and transient cognition and mood alterations) collectively referred to as sickness behavior and contributing to host defense (Capuron and Miller, 2011a; Dantzer et al, 2008). This adaptive behavioral response is supposed to be strictly tailored to the stimulus and time-limited. However, it can sometimes become abnormal and trigger neuropsychiatric symptoms, in particular when brain inflammation remains chronically activated or badly regulated (Borsini et al, 2015; Dantzer et al, 2008). Most evidence supporting these findings comes from clinical studies involving patients receiving cytokines, in particular interferon (IFN)-α, as treatment for cancers or hepatitis C. Although they are free of any psychiatric antecedent, up to 45% of these patients develop major depression during IFN-α therapy, unless they receive a prophylactic antidepressant treatment (Capuron et al, 2002; Musselman et al, 2001).
A large set of clinical and preclinical data suggest that cytokine-induced depression may rely on impairment of monoaminergic neurotransmission (particularly, serotonin, glutamate, and dopamine), likely owing to inflammation-induced activation of specific enzymes in activated monocytes, macrophages, and brain microglia (Capuron and Miller, 2011a; Capuron et al, 2011c; Dantzer et al, 2008) (Figure 2). One of these enzymes is GTP-cyclohydrolase 1 (GTP-CH1) (Felger and Miller, 2012; Haroon et al, 2012), which produces neopterin at the expense of tetrahydrobiopterin, or BH4, a co-factor essential for dopamine and serotonin biosyntheses (Capuron et al, 2011c; Murr et al, 2002; Oxenkrug et al, 2011). Consistent with the hypothesis that inflammation-induced alterations in the GTP-CH1 pathway contributes to the development of neuropsychiatric symptoms, reduced BH4 levels are reported in patients with psychiatric disorders (Hashimoto et al, 1994; Hoekstra et al, 2001). Similarly, increased blood neopterin concentrations correlate with a higher number of depressive episodes in patients with major depression (Celik et al, 2010). In addition, fatigue, decreased motivation, and anhedonia in IFN-α-treated patients and non-human primates correlate with significant alterations in BH4 (Felger et al, 2013) and dopamine metabolism/function (Capuron et al, 2012). GTP-CH1 activation also impairs nitric oxide synthase (NOS) activity, leading to generation of free radicals and oxidative stress and reducing BH4 availability by promoting its oxidation. Akin with this, systemic administration of IFN-α decreases the brain levels of dopamine and BH4 in rats through a mechanism involving NO, as this effect is reversed by treatment with an NOS inhibitor (Kitagami et al, 2003).
Inflammation induces alterations in monoamine biosynthesis. Alterations in adipose tissue and gut microbiota lead to the production of inflammatory factors and endotoxemia, thus promoting a state of chronic low-grade inflammation. Inflammatory factors activate GTP-cyclohydrolase-1 (GTP-CH1), leading to the production of neopterin at the expense of tetrahydrobiopterin (BH4) and reducing activity of the enzymes tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) involved in dopamine and serotonin syntheses. In addition, lower level of BH4 reduces nitric oxide synthase (NOS) activity and increased O2 concentration, which exacerbates BH4 inhibition. Cytokines also activate the enzyme indoleamine 2,3-dioxygenase (IDO), which results in the degradation of tryptophan along the kynurenine pathway at the expense of serotonin. Kynurenine is then degraded into quinolinic acid in microglia, thus promoting neurotoxicity.
Another enzyme also activated by cytokines and whose involvement in inflammation-induced neuropsychiatric symptoms has been well documented is the indoleamine 2,3-dioxygenase (IDO). This enzyme is the first and rate-limiting enzyme degrading tryptophan, the essential amino-acid precursor of serotonin, along the kynurenine pathway (Dantzer et al, 2008; O'Connor et al, 2009b; O'Connor et al, 2009c). By consuming tryptophan, IDO activation can therefore, in turn, reduce the synthesis of serotonin. Association of neuropsychiatric symptoms with increased kynurenine levels or altered kynurenine/tryptophan ratio has been demonstrated in several conditions associated with inflammation, including IFN-α-treated patients, aging, or Alzheimer’s disease (Capuron et al, 2011c; Gulaj et al, 2010; Forrest et al, 2011; Gold et al, 2011; Raison et al, 2010). Microglial activation of the kynurenine pathway can also ultimately lead to the production of neuroactive glutamatergic compounds, including 3-hydroxykynurenine and quinolinic acid, which have a key role in neuronal death by stimulating NMDA receptors and promoting oxidative stress (Campbell et al, 2014; Dantzer and Walker, 2014; Stone et al, 2012). In support of this notion, brain or cerebrospinal fluid concentrations of kynurenine neurotoxic metabolites are elevated in several neuropsychiatric or neurodegenerative diseases (Campbell et al, 2014; Schwarcz et al, 2001; Steiner et al, 2011; Stone et al, 2012) and have been related to the stretch of brain damages, impaired neurogenesis, and development of neuropsychiatric symptoms (Savitz et al, 2015a; Savitz et al, 2015b; Stone et al, 2012; Zunszain et al, 2012). In line with clinical findings, preclinical studies performed in rodents treated with an immune challenge have documented associations between emotional/cognitive alterations and both peripheral and brain IDO activation (Andre et al, 2008; Corona et al, 2013; Frenois et al, 2007; Gibney et al, 2013; Lawson et al, 2013; Lestage et al, 2002; Moreau et al, 2005, 2008; Salazar et al, 2012; Xie et al, 2014). In addition, aged mice or mice with constitutive microglial overactivation display, upon immune challenge, sustained cytokine production together with protracted brain IDO expression and depressive-like behavior (Corona et al, 2010; Godbout et al, 2008; Kelley et al, 2013). More importantly, pharmacological or genetic inhibition of IDO prevents the development of depressive-/anxiety-like behaviors and cognitive impairments (Barichello et al, 2013; Henry et al, 2009; O'Connor et al, 2009a; O'Connor et al, 2009b; O'Connor et al, 2009c; Salazar et al, 2012; Xie et al, 2014). Conversely, systemic kynurenine administration dose-dependently impairs emotional behaviors and spatial memory (Alexander et al, 2012; Chess et al, 2009; O'Connor et al, 2009c; Salazar et al, 2012). Of note, blockade of NMDA receptors inhibits the induction of depressive-like behavior by an immune challenge (Walker et al, 2013), whereas mice deficient for IDO are protected against NMDA receptor-mediated excitotoxicity (Mazarei et al, 2013). Altogether, these results point to a pivotal role of inflammation-induced impairment of brain neurotransmission and/or promotion of neurotoxicity in mediating the development of neuropsychiatric symptoms in inflammatory conditions.
INFLAMMATION IN DEPRESSIVE COMORBIDITIES OF OBESITY
Clinical and Experimental Evidence
Growing evidence suggests that obesity-related inflammation has a central role in the development of depressive comorbidities (Castanon et al, 2014; Lasselin and Capuron, 2014a). At the clinical level, significant associations between elevated levels of inflammatory mediators (eg, CRP, IL-6, and leptin) and depressive symptoms have been documented in individuals suffering from obesity or the metabolic syndrome (Capuron et al, 2008; Chirinos et al, 2013; Dixon et al, 2008; Ladwig et al, 2003). Moreover, in patients with the metabolic syndrome, systemic low-grade inflammation was found to represent a major determinant of depressive symptoms (Capuron et al, 2008). More recently, it was shown that CRP levels explain approximately 20% of the increase in depression scores over time in obese subjects (Daly, 2013). Consistent with the role of adiposity in these associations, reductions in levels of inflammatory markers after bariatric surgery-induced weight loss correlate with significant improvements in the emotional status and depressive symptoms of obese individuals (Capuron et al, 2011b; Emery et al, 2007). In addition, severely obese individuals have been found to display lower circulating tryptophan levels together with greater kynurenine levels in comparison to lean controls, consistent with inflammation-driven activation of IDO pathway (Brandacher et al, 2007). A shift toward activation of the enzymatic pathway leading to the production of kynurenine neurotoxic metabolites was also demonstrated in obese patients (Favennec et al, 2015). Interestingly, the recent finding that adipokines with potent anti-inflammatory properties (eg, adiponectin) have a role in the antidepressant effects of the NMDA receptor antagonist ketamine further supports the notion that inflammation-induced alterations of glutamatergic neurotransmission may contribute to the development of obesity-related depressive comorbidities (Machado-Vieira et al, 2016). Although activation of the kynurenine pathway has been primarily reported to represent a key component in the initiation and propagation of obesity and related medical complications, including CVD and the metabolic syndrome (Mangge et al, 2014), it is thus also possible that it contributes to the development of depressive comorbidities, notably through neurotoxic effects of kynurenine metabolites. The hippocampal atrophy found in obese subjects in comparison with healthy controls is in favor of this assumption (Fotuhi et al, 2012).
In line with clinical findings, recent preclinical studies have started to describe the causality of events and to identify some underlying mechanisms. The occurrence of behavioral (mood and cognitive) symptoms in DIO models or genetically obese rodents (db/db) was found to be associated with higher hippocampal and cortical expression of inflammatory cytokines (Castanon et al, 2015; Dinel et al, 2011, 2014; Erion et al, 2014; Kanoski and Davidson, 2011; Pistell et al, 2010). Interestingly, hippocampal IL-1β expression in obese db/db mice is related to adiposity and its blockade prevents cognitive impairment by normalizing dendritic spine density and local synaptic dysfunction (Erion et al, 2014). Of note, db/db mice also display association between hippocampal microglial activation and obesity-related elevation in plasma glucocorticoids (Dey et al, 2014). Similarly, DIO mice display exacerbated HPA axis activation in response to an immune challenge, together with increased neuroinflammation and depressive-like behavior (Andre et al, 2014). These results support the notion that inflammatory factors and HPA axis, which are tightly interrelated and highly activated in obesity (Dinel et al, 2011, 2014), may act together in that context to promote mood alterations (Dey et al, 2014; Hryhorczuk et al, 2013; Stranahan et al, 2008). Both cytokines and glucocorticoids have been shown to impair hippocampal neurogenesis and neuronal function in obese mice (Dinel et al, 2011; Erion et al, 2014; Stranahan et al, 2008; Wosiski-Kuhn et al, 2014). Moreover, behavioral alterations reported in obese animals are linked to increased inflammation and reduced levels of the neurotrophic factor BDNF in the cortex and hippocampus (Dinel et al, 2011; Pistell et al, 2010), whereas normalization of hippocampal BDNF levels prevents hippocampus-mediated cognitive impairments (Kariharan et al, 2015; Moy and McNay, 2013).
Taken together, these results point to a link between increased neuroinflammation, impaired neurogenesis/synaptic plasticity, and behavioral alterations in obesity. Supporting the implication of brain kynurenine pathway activation in these processes, an association was recently found between the amplitude of brain IDO activation and the development of depressive-like behavior in obese mice challenged with LPS (Andre et al, 2014; Dinel et al, 2014). Activation of GTP-CH1, as reflected by increased circulating levels of neopterin, has also been reported in obese rats (Finkelstein et al, 1982) and patients (Ledochowski et al, 1999; Oxenkrug et al, 2011; Oxenkrug, 2010). These data suggest that this activation, and the consequent alteration of dopamine neurotransmission, may also contribute to the development of neuropsychiatric symptoms in obesity. Although this assumption still needs to be confirmed, it is supported by several reports documenting impaired dopamine function together with alterations in basal ganglia and reward circuitry in obese patients (de Weijer et al, 2011; Volkow et al, 2011; Wang et al, 2001). Moreover, depressive-like behavior is associated with alterations in striatal circuitry in DIO mice, further supporting a role for dopamine-related disruptions in obesity-associated depressive symptoms (Sharma and Fulton, 2013). Finally, it is worth mentioning that, in addition to the different pathways discussed above, alterations of the gut–brain axis may represent another mechanism of inflammation-driven neuropsychiatric comorbidities, given the role of this axis in the development of neuropsychiatric symptoms after chronic stress (Cryan and Dinan, 2012; Grenham et al, 2011). The recent finding that gut microbiota transplantation from obese to lean mice is able to induce neurobehavioral changes in the absence of obesity supports this notion (Bruce-Keller et al, 2015). Altogether, these data point to brain inflammation as a major player in the development of obesity-related neuropsychiatric symptoms and start to highlight the participation of several pathways/systems that are not necessarily specific to the condition of obesity but that can still modulate or relay the brain impact of inflammatory processes.
Role of Insulin Resistance
Among the pathways by which inflammation may promote the development of neuropsychiatric comorbidities, insulin resistance, which is a trademark of severe obesity and the metabolic syndrome, deserves to receive special attention. Insulin, whose circulating levels and signaling pathway are altered in obesity, is able to interact with inflammatory processes and to act at the periphery and within the brain, in particular, in the hypothalamus, to control energy expenditure, glucose homeostasis, and feeding behavior (Hemmati et al, 2014; Hotamisligil, 2006; Velloso et al, 2008). Systemic inflammation resulting from the recruitment of macrophages in adipose tissue and pancreatic islets reported in obesity has been shown to impact β-cell secretory function and survival, reducing in turn insulin secretion and initiating insulin resistance (Donath and Shoelson, 2011; Hotamisligil, 2006). From a molecular perspective, inflammatory cytokines (in particular, IL-1β and TNF-α) have been shown to impair signaling of the insulin receptor both at the periphery and within the brain (De Souza et al, 2005; Hotamisligil, 2006; Lann and LeRoith, 2007; Xu et al, 2003). Reciprocally, emerging evidence suggests that insulin may display anti-inflammatory properties by preventing hyperglycemia and modulating key inflammatory molecules (for reviews, see Hyun et al, 2011; Scheen et al, 2015). Interestingly, recent reports on brain location of insulin receptors and their link with neuronal function and mood have introduced new ways of considering this hormone, in particular, regarding its potential role in obesity-related neuropsychiatric symptoms (Blazquez et al, 2014; Ghasemi et al, 2013a). In support of this notion, converging findings reveal dysfunctions of insulin signaling pathway in different neurological or neuropsychiatric disorders (Blazquez et al, 2014; Ghasemi et al, 2013b; Yates et al, 2012). A number of epidemiological studies also point to an association between medical conditions defined by insulin resistance and depression (Cline et al, 2012; Kan et al, 2013; Pomytkin et al, 2015). In addition, insulin resistance displayed by obese db/db mice in brain areas with high density of insulin receptors, such as the hippocampus and cortex, is associated with emotional alterations (Dey et al, 2014; Kim et al, 2011). Conversely, compounds enhancing neuronal insulin receptor-mediated transmission in the hippocampus show antidepressant-like effects in preclinical paradigms of depression (Cline et al, 2012; Cline et al, 2015). In line with these findings, recent pharmacological studies highlight the antidepressant properties of several antidiabetic drugs, which may involve, beyond improvement of hyperglycemia, positive impact on inflammation and neuronal activity (Gupta et al, 2014; Pomytkin et al, 2015). Consistent with this, normalizing hyperglycemia in db/db mice does not improve anxiety-like behavior or spatial memory deficits (Stranahan et al, 2008; Stranahan et al, 2009), whereas these are improved by reducing hippocampal inflammation (Erion et al, 2014). Moreover, treating hyperglycemic mice with the antidiabetic drug, extendin-4, has been recently shown to improve cognitive dysfunction by reducing hippocampal inflammation (Huang et al, 2012). Altogether, these data are in favor of the involvement of inflammation-related complex non-exclusive pathophysiological processes in the development of neuropsychiatric symptoms in obesity. This notion is comforted by data indicating that the risk of depressive symptoms in obese individuals is increased in metabolically unhealthy obesity, ie, when adiposity is associated with greater inflammation and metabolic abnormalities (Jokela et al, 2014).
FUTURE RESEARCH DIRECTIONS
Given the alarming and continuous rise in depressive disorders and obesity, their intricate relationship and their additive role as risk factors for many other medical complications, multiple efforts have been carried out over the past decades to identify preventive and/or therapeutic strategies aiming at reducing their health and economic impact. The complex and bidirectional relationships existing between increased adiposity and depressive disorders emphasize common pathophysiological mechanisms. As illustrated by both clinical and preclinical evidence reported in this review, these mechanisms seem to converge on inflammatory processes and related alterations of neuroendocrine and neurotransmitter pathways, which are a trademark of both disorders. Inflammation appears therefore to be the cornerstone of the different factors contributing to link depressive disorders and obesity (Figure 3). In that context, strategies to reduce inflammation, either pharmacological or non-pharmacological, may help in the prevention and management of obesity-related neuropsychiatric comorbidity and improve quality of life and health outcomes in patients afflicted with these conditions.
Overview of the mechanisms and pathways of adiposity-driven inflammation in depressive morbidity. Overweight/obesity is associated with low-grade inflammation originating from the adipose tissue and changes in gut microbiota composition. Immune-to-brain communication leads to the activation of brain inflammatory processes responsible for substantial changes in brain function, including alterations in neurotransmitter biosynthesis and changes in neurogenesis and synaptic plasticity. Concomitantly, brain cytokines promote neurotoxicity through activation of glutamate pathways. Altogether, these alterations contribute to the development of depressive symptoms. BH4: tetrahydrobiopterin; GTP-CH1: guanosine triphosphate cyclohydrolase-1; IDO: indoleamine 2,3-dioxygenase; LPS: lipopolysaccharide.
Pharmacological Strategies
Anti-inflammatory treatments that may be relevant for the prevention and management of depressive disorders occurring in conditions of overweight, obesity, or metabolic disorders may target either directly inflammatory signaling pathways or indirectly the enzymatic pathways that are modulated by inflammation. In particular, the administration of anticytokines, including the monoclonal antibody against TNF-α, infliximab, may represent a useful strategy. In support of this notion, infliximab was found to reduce depressive symptoms in rodent models of depression (Karson et al, 2013; Liu et al, 2015) as well as in humans suffering from major depression and exhibiting higher level of circulating CRP (Raison et al, 2013). Interestingly, these patients were also those who exhibited higher BMI. These promising findings highlight the necessity of clearly identifying, in future studies, the subgroups of depressed patients who could benefit from such treatment, in particular, by taking into account their metabolic and inflammatory profiles. Beyond cytokines themselves, antidepressant-like effects have been recently reported in obese mice after pharmacological blockade of cyclooxygenase-2, an enzyme activated by cytokines (Kurhe et al, 2014). On the other hand, the opportunity of blocking other enzymatic targets of cytokines, in particular, those involved in the kynurenine pathway whose activity increases with adiposity (Favennec et al, 2015) and correlates with depressive symptoms in both obese individuals and animals, likely represents another interesting therapeutic approach, as recently suggested from findings in diabetic rats (da Silva Dias et al, 2015). Alternatively, targeting the neopterin pathway, in particular, BH4, which is a critical cofactor for monoamine synthesis and is altered in obesity (Finkelstein et al, 1982; Ledochowski et al, 1999; Oxenkrug et al, 2011; Oxenkrug, 2010), may also provide a useful way to reduce adiposity-driven inflammation and related depressive symptoms. This assumption is supported by findings reporting relief of treatment refractory depression by BH4 replacement (Curtius et al, 1983; Pan et al, 2011). Finally, owing to the tight interactions between inflammation and metabolic alterations, including insulin resistance and dyslipidemia, adiposity-driven inflammation may also be targeted by antidiabetic drugs, which have already been shown to display antidepressant properties (Gupta et al, 2014; Pomytkin et al, 2015; Scheen et al, 2015). Similarly, recent clinical evidence indicates that the antidyslipidemia drugs, statins, which also display anti-inflammatory properties, improve the efficacy of antidepressant treatment when administered as adjuvant therapy (Köhler et al, 2016; Salagre et al, 2016).
Non-Pharmacological Interventions
Non-pharmacological interventions, including weight loss programs and nutritional approaches, may be of particular interest to lower inflammation and consequently improve mental health in individuals with obesity and comorbid depressive symptoms. In support of this notion, sustained weight loss, induced by low-calorie diet or bariatric surgery, was found to potently regulate inflammation (Bastard et al, 2000; Belza et al, 2009; Capuron et al, 2011b; Clement et al, 2004) and to improve depressive symptoms in obese individuals (Brinkworth et al, 2009; Capuron et al, 2011b; Dawes et al, 2016; Dixon et al, 2003; Dixon et al, 2008). Similarly, exercise, which promotes weight loss and maintenance, stimulates the production of muscular cytokines with anti-inflammatory properties (Peake et al, 2015; Pedersen et al, 2007) and was found to possess antidepressant effects comparable to antidepressant medication (Blumenthal et al, 2007; Dunn et al, 2005).
In addition, nutritional interventions based on factors with immunomodulatory properties and known impact on behavior and mood appear as tractable strategies for developing novel therapeutics for obesity-related neuropsychiatric disorders. These strategies not only include the use of omega-3 polyunsaturated fatty acids (n-3 PUFA) and antioxidants (for reviews, see Bazinet and Laye, 2014; Gomez-Pinilla and Nguyen, 2012) but also compounds that beneficially alter the microbiota (eg, prebiotics or probiotics) (Cryan and Dinan, 2012). Relevant to the role of inflammation in promoting effects of nutritional interventions on depressive symptoms, the n-3 PUFA eicosapentaenoic acid (EPA) was recently found to be effective in the prevention of IFN-α-induced depression in hepatitis C-infected patients (Su et al, 2014). In addition, a recent report indicates that patients with major depression and high level of systemic inflammation, which highly correlates with increased BMI, are more likely to respond to EPA treatment than depressed subjects with low inflammation (Rapaport et al, 2016). These findings support the hypothesis that nutritional intervention with EPA n-3 PUFA may be of particular relevance for improving depressive symptoms in obese individuals. Alternatively, the opportunity of targeting enzymatic pathways activated by cytokines and altering mood (eg, IDO/BH4 pathways) with amino-acid-based compounds also deserves to be considered.
CONCLUSIONS
Depression and overweight/obesity currently represent important public health concerns given their increasing prevalence worldwide, their societal burden, and their substantial impact on health and morbidity. Recent evidence highlights an intricate relationship between depression and obesity and suggests that the pandemic of overweight/obesity may contribute to the increased prevalence of depression. Findings discussed in the present review point to inflammation as a pivotal and crucial mediator of the relationship between adiposity and depression. The implication of such findings may contribute to the identification of novel targets for a better prevention and treatment of depression in chronic medical conditions associated with increased inflammatory processes, such as overweight/obesity and metabolic disorders.
FUNDING AND DISCLOSURE
This work was supported by subventions from the INRA, the Region Aquitaine (grant no. 2013-13-03-001, to NC), the European Community (sixth framework program) (grant no. IRG2006-039575, to LC), and the French National Research Agency, ANR (ANR-11-JSV1-0006 and ANR-13-NEUR-0004-03, both to LC). The authors declare no conflict of interest.
References
Alciati A, D'Ambrosio A, Foschi D, Corsi F, Mellado C, Angst J (2007). Bipolar spectrum disorders in severely obese patients seeking surgical treatment. J Affect Disord 101: 131–138.
Alexander KS, Wu HQ, Schwarcz R, Bruno JP (2012). Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 220: 627–637.
Almas A, Forsell Y, Iqbal R, Janszky I, Moller J (2015). Severity of depression, anxious distress and the risk of cardiovascular disease in a Swedish population-based cohort. PLoS One 10: e0140742.
Andre C, Dinel AL, Ferreira G, Laye S, Castanon N (2014). Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun 41: 10–21.
Andre C, O'Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N (2008). Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J Neuroimmunol 200: 90–99.
Anisman H, Merali Z, Hayley S (2008). Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol 85: 1–74.
Armougom F, Henry M, Vialettes B, Raccah D, Raoult D (2009). Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One 4: e7125.
Aron-Wisnewsky J, Dore J, Clement K (2012). The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol 9: 590–598.
Barichello T, Generoso JS, Simoes LR, Elias SG, Tashiro MH, Dominguini D et al (2013). Inhibition of indoleamine 2,3-dioxygenase prevented cognitive impairment in adult Wistar rats subjected to pneumococcal meningitis. Transl Res 162: 390–397.
Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M et al (2000). Elevated levels of interleukin-6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 85: 3338–3342.
Bazinet RP, Laye S (2014). Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15: 771–785.
Belza A, Toubro S, Stender S, Astrup A (2009). Effect of diet-induced energy deficit and body fat reduction on high-sensitive CRP and other inflammatory markers in obese subjects. Int J Obes (Lond) 33: 456–464.
Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D et al (2003). A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA 100: 1920–1925.
Blazquez E, Velazquez E, Hurtado-Carneiro V, Ruiz-Albusac JM (2014). Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front Endocrinol (Lausanne) 5: 161.
Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA et al (2007). Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 69: 587–596.
Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S et al (2014). Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun 40: 9–17.
Borsini A, Zunszain PA, Thuret S, Pariante CM (2015). The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38: 145–157.
Brandacher G, Hoeller E, Fuchs D, Weiss HG (2007). Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab 8: 289–295.
Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ (2009). Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med 169: 1873–1880.
Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard Et, Taylor CM, Welsh DA et al (2015). Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77: 607–615.
Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G et al (2007). Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 292: G518–G525.
Brunault P, Jacobi D, Miknius V, Bourbao-Tournois C, Huten N, Gaillard P et al (2012). High preoperative depression, phobic anxiety, and binge eating scores and low medium-term weight loss in sleeve gastrectomy obese patients: a preliminary cohort study. Psychosomatics 53: 363–370.
Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK et al (2013). Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun 35: 33–42.
Cai D, Liu T (2012). Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging (Albany NY) 4: 98–115.
Campbell BM, Charych E, Lee AW, Moller T (2014). Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 8: 12.
Cancello R, Clement K (2006). Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 113: 1141–1147.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM et al (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481.
Cani PD, Osto M, Geurts L, Everard A (2012). Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3: 279–288.
Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O et al (2009). Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103.
Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB et al (2002). Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26: 643–652.
Capuron L, Miller AH (2011a). Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130: 226–238.
Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ et al (2012). Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69: 1044–1053.
Capuron L, Poitou C, Machaux-Tholliez D, Frochot V, Bouillot JL, Basdevant A et al (2011b). Relationship between adiposity, emotional status and eating behaviour in obese women: role of inflammation. Psychol Med 41: 1517–1528.
Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P et al (2011c). Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry 70: 175–182.
Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ et al (2008). Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry 64: 896–900.
Carpenter KM, Hasin DS, Allison DB, Faith MS (2000). Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health 90: 251–257.
Castanon N, Lasselin J, Capuron L (2014). Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol (Lausanne) 5: 74.
Castanon N, Luheshi G, Laye S (2015). Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front Neurosci 9: 229.
Castanon N, Medina C, Mormede C, Dantzer R (2004). Chronic administration of tianeptine balances lipopolysaccharide-induced expression of cytokines in the spleen and hypothalamus of rats. Psychoneuroendocrinology 29: 778–790.
Celik C, Erdem M, Cayci T, Ozdemir B, Ozgur Akgul E, Kurt YG et al (2010). The association between serum levels of neopterin and number of depressive episodes of major depression. Prog Neuropsychopharmacol Biol Psychiatry 34: 372–375.
Chess AC, Landers AM, Bucci DJ (2009). L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res 201: 325–331.
Chirinos DA, Goldberg R, Gellman M, Mendez AJ, Gutt M, McCalla JR et al (2013). Leptin and its association with somatic depressive symptoms in patients with the metabolic syndrome. Ann Behav Med 46: 31–39.
Chou KL, Yu KM (2013). Atypical depressive symptoms and obesity in a national sample of older adults with major depressive disorder. Depress Anxiety 30: 574–579.
Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB et al (2010). A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry 67: 1075–1082.
Cizza G, Ronsaville DS, Kleitz H, Eskandari F, Mistry S, Torvik S et al (2012). Clinical subtypes of depression are associated with specific metabolic parameters and circadian endocrine profiles in women: the power study. PLoS One 7: e28912.
Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA et al (2004). Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 18: 1657–1669.
Cline BH, Costa-Nunes JP, Cespuglio R, Markova N, Santos AI, Bukhman YV et al (2015). Dicholine succinate, the neuronal insulin sensitizer, normalizes behavior, REM sleep, hippocampal pGSK3 beta and mRNAs of NMDA receptor subunits in mouse models of depression. Front Behav Neurosci 9: 37.
Cline BH, Steinbusch HW, Malin D, Revishchin AV, Pavlova GV, Cespuglio R et al (2012). The neuronal insulin sensitizer dicholine succinate reduces stress-induced depressive traits and memory deficit: possible role of insulin-like growth factor 2. BMC Neurosci 13: 110.
Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG et al (2010). Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation 7: 93.
Corona AW, Norden DM, Skendelas JP, Huang Y, O'Connor JC, Lawson M et al (2013). Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun 31: 134–142.
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E et al (2013). Dietary intervention impact on gut microbial gene richness. Nature 500: 585–588.
Crump C, Sundquist K, Winkleby MA, Sundquist J (2013). Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry 70: 931–939.
Cryan JF, Dinan TG (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13: 701–712.
Curtius HC, Niederwieser A, Levine RA, Lovenberg W, Woggon B, Angst J (1983). Successful treatment of depression with tetrahydrobiopterin. Lancet 1: 657–658.
da Silva Dias IC, Carabelli B, Ishii DK, de Morais H, de Carvalho MC, Rizzo de Souza LE et al (2015). Indoleamine-2,3-dioxygenase/kynurenine pathway as a potential pharmacological target to treat depression associated with diabetes. Mol Neurobiol (in press).
Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A et al (2001). Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol 81: E326–E334.
Daly M (2013). The relationship of C-reactive protein to obesity-related depressive symptoms: a longitudinal study. Obesity 21: 248–250.
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56.
Dantzer R, Walker AK (2014). Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J Neural Transm (Vienna) 121: 925–932.
Dawes AJ, Maggard-Gibbons M, Maher AR, Booth MJ, Miake-Lye I, Beroes JM et al (2016). Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA 315: 150–163.
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC et al (2005). Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199.
de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM et al (2011). Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res 1: 37.
de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P (2010). Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res 178: 230–235.
Dey A, Hao S, Erion JR, Wosiski-Kuhn M, Stranahan AM (2014). Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J Neuroimmunol 269: 20–27.
Dinel AL, Andre C, Aubert A, Ferreira G, Laye S, Castanon N (2011). Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One 6: e24325.
Dinel AL, Andre C, Aubert A, Ferreira G, Laye S, Castanon N (2014). Lipopolysaccharide-induced brain activation of the indoleamine 2,3-dioxygenase and depressive-like behavior are impaired in a mouse model of metabolic syndrome. Psychoneuroendocrinology 40: 48–59.
Dixon JB, Dixon ME, O'Brien PE (2003). Depression in association with severe obesity: changes with weight loss. Arch Intern Med 163: 2058–2065.
Dixon JB, Hayden MJ, Lambert GW, Dawood T, Anderson ML, Dixon ME et al (2008). Raised CRP levels in obese patients: symptoms of depression have an independent positive association. Obesity 16: 2010–2015.
Donath MY, Shoelson SE (2011). Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98–107.
Duarte-Guerra LS, Coelho BM, Santo MA, Wang YP (2015). Psychiatric disorders among obese patients seeking bariatric surgery: results of structured clinical interviews. Obes Surg 25 5: 830–837.
Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO (2005). Exercise treatment for depression: efficacy and dose response. Am J Prev Med 28: 1–8.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M et al (2005). Diversity of the human intestinal microbial flora. Science 308: 1635–1638.
Emery CF, Fondow MD, Schneider CM, Christofi FL, Hunt C, Busby AK et al (2007). Gastric bypass surgery is associated with reduced inflammation and less depression: a preliminary investigation. Obes Surg 17: 759–763.
Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK et al (2014). Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci 34: 2618–2631.
Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR et al (2005). Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry 58: 175–189.
Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M et al (2015). The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 23: 2066–2074.
Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL et al (2013). Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav Immun 31: 153–160.
Felger JC, Miller AH (2012). Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 33: 315–327.
Finelli C, Padula MC, Martelli G, Tarantino G (2014). Could the improvement of obesity-related co-morbidities depend on modified gut hormones secretion? World J Gastroenterol 20: 16649–16664.
Finkelstein JA, Chance WT, Fischer JE (1982). Brain serotonergic activity and plasma amino acid levels in genetically obese Zucker rats. Pharmacol Biochem Behav 17: 939–944.
Flint HJ (2011). Obesity and the gut microbiota. J Clin Gastroenterol 45 (Suppl): S128–S132.
Forrest CM, Mackay GM, Oxford L, Millar K, Darlington LG, Higgins MJ et al (2011). Kynurenine metabolism predicts cognitive function in patients following cardiac bypass and thoracic surgery. J Neurochem 119: 136–152.
Fotuhi M, Do D, Jack C (2012). Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8: 189–202.
Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J et al (2007). Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32: 516–531.
Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL et al (2010). Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59: 3049–3057.
Gao Y, Ottaway N, Schriever SC, Legutko B, Garcia-Caceres C, de la Fuente E et al (2014). Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62: 17–25.
Ghasemi R, Dargahi L, Haeri A, Moosavi M, Mohamed Z, Ahmadiani A (2013a). Brain insulin dysregulation: implication for neurological and neuropsychiatric disorders. Mol Neurobiol 47: 1045–1065.
Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A (2013b). Insulin in the brain: sources, localization and functions. Mol Neurobiol 47: 145–171.
Gibney SM, McGuinness B, Prendergast C, Harkin A, Connor TJ (2013). Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun 28: 170–181.
Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O'Connor J et al (2008). Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology 33: 2341–2351.
Gold AB, Herrmann N, Swardfager W, Black SE, Aviv RI, Tennen G et al (2011). The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. J Neuroinflammation 8: 17.
Gomez-Pinilla F, Nguyen TT (2012). Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutr Neurosci 15: 127–133.
Gregor MF, Hotamisligil GS (2011). Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445.
Grenham S, Clarke G, Cryan JF, Dinan TG (2011). Brain-gut-microbe communication in health and disease. Front Physiol 2: 94.
Grothe KB, Mundi MS, Himes SM, Sarr MG, Clark MM, Geske JR et al (2014). Bipolar disorder symptoms in patients seeking bariatric surgery. Obes Surg 24: 1909–1914.
Gulaj E, Pawlak K, Bien B, Pawlak D (2010). Kynurenine and its metabolites in Alzheimer's disease patients. Adv Med Sci 55: 204–211.
Gupta D, Kurhe Y, Radhakrishnan M (2014). Antidepressant effects of insulin in streptozotocin induced diabetic mice: modulation of brain serotonin system. Physiol Behav 129: 73–78.
Hakeam HA, O'Regan PJ, Salem AM, Bamehriz FY, Jomaa LF (2009). Inhibition of C-reactive protein in morbidly obese patients after laparoscopic sleeve gastrectomy. Obes Surg 19: 456–460.
Hammond RA, Levine R (2010). The economic impact of obesity in the United States. Diabetes Metab Syndr Obes 3: 285–295.
Hare DL, Toukhsati SR, Johansson P, Jaarsma T (2014). Depression and cardiovascular disease: a clinical review. Eur Heart J 35: 1365–1372.
Haroon E, Raison CL, Miller AH (2012). Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37: 137–162.
Hashimoto R, Mizutani M, Ohta T, Nakazawa K, Nagatsu T (1994). Changes in plasma tetrahydrobiopterin levels of depressives in depressive and remission phases: reconfirmed by measurement with an internal standard. Neuropsychobiology 29: 57–60.
Hasler G, Pine DS, Gamma A, Milos G, Ajdacic V, Eich D et al (2004). The associations between psychopathology and being overweight: a 20-year prospective study. Psychol Med 34: 1047–1057.
Hemmati F, Ghasemi R, Mohamed Ibrahim N, Dargahi L, Mohamed Z, Raymond AA, Ahmadiani A (2014). Crosstalk between insulin and Toll-like receptor signaling pathways in the central nervous system. Mol Neurobiol 50: 797–810.
Henry CJ, Huang Y, Wynne AM, Godbout JP (2009). Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 23: 309–317.
Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS (2006). Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes (Lond) 30: 513–519.
Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Laksy K et al (2006). Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes (Lond) 30: 520–527.
Hoekstra R, van den Broek WW, Fekkes D, Bruijn JA, Mulder PG, Pepplinkhuizen L (2001). Effect of electroconvulsive therapy on biopterin and large neutral amino acids in severe, medication-resistant depression. Psychiatry Res 103: 115–123.
Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature 444: 860–867.
Hryhorczuk C, Sharma S, Fulton SE (2013). Metabolic disturbances connecting obesity and depression. Front Neurosci 7: 177.
Huang HJ, Chen YH, Liang KC, Jheng YS, Jhao JJ, Su MT et al (2012). Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS One 7: e39656.
Hyun E, Ramachandran R, Hollenberg MD, Vergnolle N (2011). Mechanisms behind the anti-inflammatory actions of insulin. Crit Rev Immunol 31: 307–340.
Janowitz D, Wittfeld K, Terock J, Freyberger HJ, Hegenscheid K, Völzke H et al (2015). Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. Neuroimage 122: 149–157.
Jokela M, Hamer M, Singh-Manoux A, Batty GD, Kivimaki M (2014). Association of metabolically healthy obesity with depressive symptoms: pooled analysis of eight studies. Mol Psychiatry 19: 910–914.
Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D et al (2013). A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care 36: 480–489.
Kanneganti TD, Dixit VD (2012). Immunological complications of obesity. Nat Immunol 13: 707–712.
Kanoski SE, Davidson TL (2011). Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 103: 59–68.
Kariharan T, Nanayakkara G, Parameshwaran K, Bagasrawala I, Ahuja M, Abdel-Rahman E et al (2015). Central activation of PPAR-gamma ameliorates diabetes induced cognitive dysfunction and improves BDNF expression. Neurobiol Aging 36: 1451–1461.
Karson A, Demirtas T, Bayramgurler D, Balci F, Utkan T (2013). Chronic administration of infliximab (TNF-alpha inhibitor) decreases depression and anxiety-like behaviour in rat model of chronic mild stress. Basic Clin Pharmacol Toxicol 112: 335–340.
Kasen S, Cohen P, Chen H, Must A (2008). Obesity and psychopathology in women: a three decade prospective study. Int J Obes (Lond) 32: 558–566.
Kelley KW, O'Connor JC, Lawson MA, Dantzer R, Rodriguez-Zas SL, McCusker RH (2013). Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guerin. Brain Behav Immun 32: 63–69.
Kendler KS, Karkowski LM, Prescott CA (1999). Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156: 837–841.
Kim B, Sullivan KA, Backus C, Feldman EL (2011). Cortical neurons develop insulin resistance and blunted Akt signaling: a potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid Redox Signal 14: 1829–1839.
Kim D, Kim J, Yoon JH, Ghim J, Yea K, Song P et al (2014). CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia 57: 1456–1465.
Kinzl JF, Schrattenecker M, Traweger C, Mattesich M, Fiala M, Biebl W (2006). Psychosocial predictors of weight loss after bariatric surgery. Obes Surg 16: 1609–1614.
Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T (2003). Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res 978: 104–114.
Kleinridders A, Konner AC, Bruning JC (2009). CNS-targets in control of energy and glucose homeostasis. Curr Opin Pharmacol 9: 794–804.
Kloiber S, Ising M, Reppermund S, Horstmann S, Dose T, Majer M et al (2007). Overweight and obesity affect treatment response in major depression. Biol Psychiatry 62: 321–326.
Köhler O, Gasse C, Petersen L, Ingstrup KG, Nierenberg AA, Mors O et al (2016). The effect of concomitant treatment with SSRIs and statins: a population-based study. Am J Psychiatry (in press).
Kurhe Y, Mahesh R, Gupta D (2014). Effect of a selective cyclooxygenase type 2 inhibitor celecoxib on depression associated with obesity in mice: an approach using behavioral tests. Neurochem Res 39: 1395–1402.
Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Koenig W (2003). Influence of depressive mood on the association of CRP and obesity in 3205 middle aged healthy men. Brain Behav Immun 17: 268–275.
Lamers F, de Jonge P, Nolen WA, Smit JH, Zitman FG, Beekman AT et al (2010). Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 71: 1582–1589.
Lann D, LeRoith D (2007). Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am 91: 1063–1077, viii.
Lasselin J, Capuron L (2014a). Chronic low-grade inflammation in metabolic disorders: relevance for behavioral symptoms. Neuroimmunomodulation 21: 95–101.
Lasselin J, Magne E, Beau C, Ledaguenel P, Dexpert S, Aubert A et al (2014b). Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery-induced weight loss. J Clin Endocrinol Metab 99: E53–E61.
Lasserre AM, Glaus J, Vandeleur CL, Marques-Vidal P, Vaucher J, Bastardot F et al (2014). Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: a prospective, population-based study. JAMA Psychiatry 71: 880–888.
Lawrence CB, Brough D, Knight EM (2012). Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Dis Model Mech 5: 649–659.
Lawson MA, McCusker RH, Kelley KW (2013). Interleukin-1 beta converting enzyme is necessary for development of depression-like behavior following intracerebroventricular administration of lipopolysaccharide to mice. J Neuroinflammation 10: 54.
Laye S, Parnet P, Goujon E, Dantzer R (1994). Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res 27: 157–162.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G et al (2013). Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546.
Ledochowski M, Murr C, Widner B, Fuchs D (1999). Association between insulin resistance, body mass and neopterin concentrations. Clin Chim Acta 282: 115–123.
Lee SH, Paz-Filho G, Mastronardi C, Licinio J, Wong ML (2016). Is increased antidepressant exposure a contributory factor to the obesity pandemic? Transl Psychiatry 6: e759.
Lestage J, Verrier D, Palin K, Dantzer R (2002). The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun 16: 596–601.
Levitan RD, Davis C, Kaplan AS, Arenovich T, Phillips DI, Ravindran AV (2012). Obesity comorbidity in unipolar major depressive disorder: refining the core phenotype. J Clin Psychiatry 73: 1119–1124.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2005). Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075.
Liu B, Kuang L, Liu J (2014). Bariatric surgery relieves type 2 diabetes and modulates inflammatory factors and coronary endothelium eNOS/iNOS expression in db/db mice. Can J Physiol Pharmacol 92: 70–77.
Liu YN, Peng YL, Liu L, Wu TY, Zhang Y, Lian YJ et al (2015). TNFalpha mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw 26: 15–25.
Lojko D, Buzuk G, Owecki M, Ruchala M, Rybakowski JK (2015). Atypical features in depression: association with obesity and bipolar disorder. J Affect Disord 185: 76–80.
Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW et al (2010). Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 67: 220–229.
Ma J, Xiao L (2010). Obesity and depression in US women: results from the 2005-2006 National Health and Nutritional Examination Survey. Obesity 18: 347–353.
Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID et al (2016). The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry; doi:10.1038/mp.2016.36 (in press).
Manco M, Fernandez-Real JM, Equitani F, Vendrell J, Valera Mora ME, Nanni G et al (2007). Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women. J Clin Endocrinol Metab 92: 483–490.
Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, Prassl R et al (2014). Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity 22: 195–201.
Mansur RB, Rizzo LB, Santos CM, Asevedo E, Cunha GR, Noto MN et al (2016). Adipokines, metabolic dysfunction and illness course in bipolar disorder. J Psychiatr Res 74: 63–69.
Maric T, Woodside B, Luheshi GN (2014). The effects of dietary saturated fat on basal hypothalamic neuroinflammation in rats. Brain Behav Immun 36: 35–45.
Martin DJ, Ul-Haq Z, Nicholl BI, Cullen B, Evans J, Gill JM et al (2016). Cardiometabolic disease and features of depression and bipolar disorder: population-based, cross-sectional study. Br J Psychiatry 208: 343–351.
Mather AA, Cox BJ, Enns MW, Sareen J (2009). Associations of obesity with psychiatric disorders and suicidal behaviors in a nationally representative sample. J Psychosom Res 66: 277–285.
Mazarei G, Budac DP, Lu G, Lee H, Moller T, Leavitt BR (2013). The absence of indoleamine 2,3-dioxygenase expression protects against NMDA receptor-mediated excitotoxicity in mouse brain. Exp Neurol 249: 144–148.
Mazzeschi C, Pazzagli C, Buratta L, Reboldi GP, Battistini D, Piana N et al (2012). Mutual interactions between depression/quality of life and adherence to a multidisciplinary lifestyle intervention in obesity. J Clin Endocrinol Metab 97: E2261–E2265.
McIntyre RS, Danilewitz M, Liauw SS, Kemp DE, Nguyen HT, Kahn LS et al (2010). Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord 126: 366–387.
McNelis JC, Olefsky JM (2014). Macrophages, immunity, and metabolic disease. Immunity 41: 36–48.
Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA et al (2016). Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 53: 39–48.
Milaneschi Y, Lamers F, Bot M, Drent ML, Penninx BW (2015). Leptin dysregulation is specifically associated with major depression with atypical features: evidence for a mechanism connecting obesity and depression. Biol Psychiatry. doi:10.1016/j.biopsych.2015.10.023 (in press).
Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE et al (2009). Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29: 359–370.
Mitchell JE, Selzer F, Kalarchian MA, Devlin MJ, Strain GW, Elder KA et al (2012). Psychopathology before surgery in the longitudinal assessment of bariatric surgery-3 (LABS-3) psychosocial study. Surg Obes Relat Dis 8: 533–541.
Moreau M, Andre C, O'Connor JC, Dumich SA, Woods JA, Kelley KW et al (2008). Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun 22: 1087–1095.
Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R et al (2005). Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis 192: 537–544.
Moy GA, McNay EC (2013). Caffeine prevents weight gain and cognitive impairment caused by a high-fat diet while elevating hippocampal BDNF. Physiol Behav 109: 69–74.
Murr C, Widner B, Wirleitner B, Fuchs D (2002). Neopterin as a marker for immune system activation. Curr Drug Metab 3: 175–187.
Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS et al (2001). Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 344: 961–966.
Nigatu YT, Bultmann U, Reijneveld SA (2015). The prospective association between obesity and major depression in the general population: does single or recurrent episode matter? BMC Public Health 15: 350.
O'Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J et al (2009a). Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci 29: 4200–4209.
O'Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J et al (2009b). Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol 182: 3202–3212.
O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N et al (2009c). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14: 511–522.
Oskooilar N, Wilcox CS, Tong ML, Grosz DE (2009). Body mass index and response to antidepressants in depressed research subjects. J Clin Psychiatry 70: 1609–1610.
Oxenkrug G, Tucker KL, Requintina P, Summergrad P (2011). Neopterin, a marker of interferon-gamma-inducible inflammation, correlates with pyridoxal-5'-phosphate, waist circumference, HDL-cholesterol, insulin resistance and mortality risk in adult Boston community dwellers of Puerto Rican origin. Am J Neuroprot Neuroregen 3: 48–52.
Oxenkrug GF (2010). Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann NY Acad Sci 1199: 1–14.
Pan L, McKain BW, Madan-Khetarpal S, McGuire M, Diler RS, Perel JM et al (2011). GTP-cyclohydrolase deficiency responsive to sapropterin and 5-HTP supplementation: relief of treatment-refractory depression and suicidal behaviour. BMJ Case Rep pii: bcr0320113927.
Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA (2006). Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 31: 1419–1425.
Park HS, Park JY, Yu R (2005). Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 69: 29–35.
Peake JM, Della Gatta P, Suzuki K, Nieman DC (2015). Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev 21: 8–25.
Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP (2007). Role of myokines in exercise and metabolism. J Appl Physiol (1985) 103: 1093–1098.
Pedersen BK, Febbraio MA (2012). Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465.
Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N (2013). Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 11: 129.
Petry NM, Barry D, Pietrzak RH, Wagner JA (2008). Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med 70: 288–297.
Pickering RP, Grant BF, Chou SP, Compton WM (2007). Are overweight, obesity, and extreme obesity associated with psychopathology? Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry 68: 998–1009.
Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK et al (2010). Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 219: 25–32.
Pohl J, Woodside B, Luheshi GN (2009). Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology 150: 4901–4910.
Pomytkin IA, Cline BH, Anthony DC, Steinbusch HW, Lesch KP, Strekalova T (2015). Endotoxaemia resulting from decreased serotonin tranporter (5-HTT) function: a reciprocal risk factor for depression and insulin resistance? Behav Brain Res 276: 111–117.
Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G et al (2010). CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15: 393–403.
Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF et al (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry 70: 31–41.
Ransohoff RM, Perry VH (2009). Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27: 119–145.
Rao SR (2012). Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res 61: 789–807.
Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R et al (2016). Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry 21: 71–79.
Rizvi SJ, Grima E, Tan M, Rotzinger S, Lin P, McIntyre RS et al (2014). Treatment-resistant depression in primary care across Canada. Can J Psychiatry 59: 349–357.
Rummel C, Inoue W, Poole S, Luheshi GN (2010). Leptin regulates leukocyte recruitment into the brain following systemic LPS-induced inflammation. Mol Psychiatry 15: 523–534.
Salagre E, Fernandes BS, Dodd S, Brownstein DJ, Berk M (2016). Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord 200: 235–242.
Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O'Connor JC (2012). Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav 62: 202–209.
Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J et al (2015a). Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology 52: 200–211.
Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS et al (2015b). Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40: 463–471.
Savitz JB, Price JL, Drevets WC (2014). Neuropathological and neuromorphometric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neurosci Biobehav Rev 42: 132–147.
Scheen AJ, Esser N, Paquot N (2015). Antidiabetic agents: potential anti-inflammatory activity beyond glucose control. Diabetes Metab 41: 183–194.
Schmidt MI, Duncan BB (2003). Diabesity: an inflammatory metabolic condition. Clin Chem Lab Med 41: 1120–1130.
Schneck AS, Iannelli A, Patouraux S, Rousseau D, Bonnafous S, Bailly-Maitre B et al (2014). Effects of sleeve gastrectomy in high fat diet-induced obese mice: respective role of reduced caloric intake, white adipose tissue inflammation and changes in adipose tissue and ectopic fat depots. Surg Endosc 28: 592–602.
Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC (2001). Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50: 521–530.
Sharma S, Fulton S (2013). Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond) 37: 382–389.
Simon GE, Ludman EJ, Linde JA, Operskalski BH, Ichikawa L, Rohde P et al (2008). Association between obesity and depression in middle-aged women. Gen Hosp Psychiatry 30: 32–39.
Sinha R, Jastreboff AM (2013). Stress as a common risk factor for obesity and addiction. Biol Psychiatry 73: 827–835.
Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z et al (2011). Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 8: 94.
Stone TW, Forrest CM, Stoy N, Darlington LG (2012). Involvement of kynurenines in Huntington's disease and stroke-induced brain damage. J Neural Transm 119: 261–274.
Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP (2008). Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 11: 309–317.
Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG et al (2009). Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 19: 951–961.
Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP et al (2014). Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry 76: 559–566.
Tehrani AB, Nezami BG, Gewirtz A, Srinivasan S (2012). Obesity and its associated disease: a role for microbiota? Neurogastroenterol Motil 24: 305–311.
Thaler JP, Schwartz MW (2010). Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151: 4109–4115.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO et al (2012). Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE et al (2009). A core gut microbiome in obese and lean twins. Nature 457: 480–484.
Vannucchi G, Toni C, Maremmani I, Perugi G (2014). Does obesity predict bipolarity in major depressive patients? J Affect Disord 155: 118–122.
Velloso LA, Araujo EP, de Souza CT (2008). Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation 15: 189–193.
Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW et al (2013). Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 21: E607–E615.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB (1999). Elevated C-reactive protein levels in overweight and obese adults. JAMA 282: 2131–2135.
Volkow ND, Wang GJ, Baler RD (2011). Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15: 37–46.
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B et al (2013). NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38: 1609–1616.
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al (2001). Brain dopamine and obesity. Lancet 357: 354–357.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE et al (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586.
Woo YS, Seo HJ, McIntyre RS, Bahk WM (2016). Obesity and its potential effects on antidepressant treatment outcomes in patients with depressive disorders: a literature review. Int J Mol Sci. pii: E80.
Wosiski-Kuhn M, Erion JR, Gomez-Sanchez EP, Gomez-Sanchez CE, Stranahan AM (2014). Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology 42: 165–177.
Wulsin LR, Singal BM (2003). Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 65: 201–210.
Xie W, Cai L, Yu Y, Gao L, Xiao L, He Q et al (2014). Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J Neuroinflammation 11: 41.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830.
Yang PJ, Lee WJ, Tseng PH, Lee PH, Lin MT, Yang WS (2014). Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surg Obes Relat Dis 10: 1182–1187.
Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A (2012). Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol 32: 2060–2067.
Yokum S, Ng J, Stice E (2012). Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond) 36: 656–664.
Zeyda M, Huber J, Prager G, Stulnig TM (2011). Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity 19: 743–748.
Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C (2011). Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol 31: 2063–2069.
Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D (2008). Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73.
Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM et al (2012). Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37: 939–949.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capuron, L., Lasselin, J. & Castanon, N. Role of Adiposity-Driven Inflammation in Depressive Morbidity. Neuropsychopharmacol 42, 115–128 (2017). https://doi.org/10.1038/npp.2016.123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.123
This article is cited by
-
Correlation analysis between physical activity and depressive tendencies among occupational groups: an isotemporal substitution approach
BMC Public Health (2023)
-
Debt Problem of One Partner and Depressive Morbidity in the Other: A 2-Year Follow-up Register Study of Different-Sex Couples in Sweden
Journal of Family and Economic Issues (2023)
-
Association between obesity and common mental disorders in women: a population-based study in Southern Brazil
Social Psychiatry and Psychiatric Epidemiology (2023)
-
The role of alcohol use and adiposity in serum levels of IL-1RA in depressed patients
BMC Psychiatry (2022)
-
Salivary bacterial signatures in depression-obesity comorbidity are associated with neurotransmitters and neuroactive dipeptides
BMC Microbiology (2022)