Abstract
The consequences of chronic stress on brain structure and function are far reaching. Whereas stress can produce short-term adaptive changes in the brain, chronic stress leads to long-term maladaptive changes that increase vulnerability to psychiatric disorders, such as anxiety and addiction. These two disorders are the most prevalent psychiatric disorders in the United States, and are typically chronic, disabling, and highly comorbid. Emerging evidence implicates a tiny brain region—the bed nucleus of the stria terminalis (BNST)—in the body’s stress response and in anxiety and addiction. Rodent studies provide compelling evidence that the BNST plays a central role in sustained threat monitoring, a form of adaptive anxiety, and in the withdrawal and relapse stages of addiction; however, little is known about the role of BNST in humans. Here, we review current evidence for BNST function in humans, including evidence for a role in the production of both adaptive and maladaptive anxiety. We also review preliminary evidence of the role of BNST in addiction in humans. Together, these studies provide a foundation of knowledge about the role of BNST in adaptive anxiety and stress-related disorders. Although the field is in its infancy, future investigations of human BNST function have tremendous potential to illuminate mechanisms underlying stress-related disorders and identify novel neural targets for treatment.
Similar content being viewed by others
INTRODUCTION
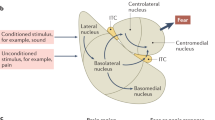
We are on the cusp of a scientific revolution that will uncover the pathophysiological basis of stress-related disorders. Emerging evidence suggests that a tiny region in the ventral forebrain—the bed nucleus of the stria terminalis (BNST)—is a critical node in the stress response neurocircuitry and may play a significant role in both anxiety and addiction, two highly prevalent and debilitating stress-related disorders. Rodent studies of the BNST over the past two decades have led to exciting discoveries about its potential role in human psychopathology—seminal studies have shown a unique role for the BNST in contextual fear and sustained, anxiety-like responses in rodents (see, eg, Davis et al, 2010; Walker et al, 2003), and parallel work in drug addiction demonstrates the role of BNST in withdrawal related-anxiety and relapse (Buffalari and See, 2011; Silberman et al, 2013; Wenzel et al, 2014). Although anxiety and addiction are often considered to have distinct neurobiological bases—anxiety related to fear neurocircuitry and addiction related to reward circuitry—we now understand the two disorders also have many commonalities; for example, anxiety and addiction are both triggered by stress (Koob, 2009; McEwen, 2012), risk for both disorders is heightened in individuals with altered stress reactivity (Lovallo, 2006; Villada et al, 2014), and the two disorders are highly comorbid (Grant et al, 2004; Kessler et al, 2005b). The BNST is ideally situated to instigate allostatic changes in the brain through its dense connections with the paraventricular nucleus (PVN) of the hypothalamus, the node of the hypothalamic–pituitary–adrenal (HPA) axis that initiates cortisol responses. Indeed, BNST lesions alter stress-related cortisol release (Sullivan et al, 2004), suggesting that the BNST may play an important role in disorders triggered by stress response, including anxiety and addiction (Figure 1).
Human anxiety and addiction circuits. The BNST is a central node in both anxiety and addiction neurocircuitry. (left) The BNST is centrally located to influence human anxiety responses, with connections to multiple limbic and brainstem regions that mediate defensive response to threat, including the amygdala, anterior insula, hippocampus, hypothalamus, and periaqueductal gray (adapted from Grupe and Nitschke, 2013). (right) The BNST is engaged during the negative emotional stage of withdrawal and interacts with the amygdala and ventral striatum, including the shell of the nucleus accumbens and ventral tegmental area, to mediate negative reinforcement (adapted from Koob and Volkow, 2010). AI, anterior insula; Amyg, amygdala; BNST, bed nucleus of the stria terminalis; Hy, hypothalamus; PAG, periaqueductal gray; VS, ventral striatum.
Despite compelling findings in rodents, the BNST has been remarkably understudied in humans. Recent advances in methods and technology have provided the necessary foundation for human BNST studies and exciting new data are emerging. Here we provide a comprehensive review of BNST structure and function in humans, including a meta-analysis of human BNST functional studies, and place current human BNST research in the context of rodent and non-human primate findings. Although the majority of current human studies examine normative BNST function in healthy controls—providing a crucial foundation for identifying alterations in BNST function—we also review preliminary evidence for the involvement of the BNST in anxiety disorders and addiction in humans. We are excited to review this field during its infancy and hope that we can inspire other researchers to investigate the BNST. The next decade promises to bring tremendous advances in knowledge; toward this end, we provide recommendations for future directions.
BUILDING BLOCKS FOR HUMAN STUDIES: CURRENT EVIDENCE IN RODENTS AND NON-HUMAN PRIMATES
The BNST Is a Core Neural Substrate of Anxiety
The past 25 years marked a period of major advances in our understanding of the unique behaviors and underlying neural substrates that distinguish among different patterns of fear responding including short-term, phasic responses to immediate threat (akin to human states of fear) and longer-term, sustained responses to more diffuse or unpredictable threats (akin to human states of anxiety) (for review see Davis et al, 2010; Walker et al, 2003). Early in this period, studies of rodent defensive behaviors provided the first evidence that defensive behaviors differed along key dimensions in ways that may be analogous to fear and anxiety in humans (Blanchard et al, 1993; Bolles and Fanselow, 1980; Fanselow, 1986). Specifically, rodents display three distinct defensive behavior patterns based on physical distance to threat: circa-strike; postencounter; and preencounter behaviors. Circa-strike behaviors occur when a threat is imminent and proximal, are characterized by a short-term response to a real and present danger, and are thought to generally reflect the adaptive state of fear. Circa-strike behaviors appear rapidly when a threat becomes imminent but also dissipate rapidly after the threat is removed. In contrast, postencounter behaviors (where threat is present but physically distant) and preencounter behaviors (in locations where threat has been previously encountered) are evoked by potential or unpredictable threat and characterized by a sustained hypervigilant response, thought to generally reflect anxiety. Thus, although short-term immediate (‘fear’) responses occur only in the immediate presence of a threat, sustained hypervigilant (‘anxiety’) responses occur both when a threat is distant and in contexts associated with threat, even when a threat is not currently present. As a predator moves closer and threat becomes more imminent, rodent defensive behavior shifts from the anxiety-like sustained threat monitoring and hypervigilance that characterize the pre- and postencounter stages to the fear-like fight or flight defensive behaviors specific to the circa-strike stage.
The recognition that varying the distance or context of threat produced distinct behaviors in rodents was intriguing and set the stage for major advances in our understanding of the neural circuitry underlying contextually-dependent threat response. Much of the initial research on the neurobiology of response to threat behaviors focused on the role of the amygdala; the distinct role of the BNST is a more recent discovery. For example, early work by Davis and colleagues (Davis et al, 1993; Lee and Davis, 1997) showed that central amygdala (CeA) lesions abolished conditioned fear response but did not affect the anxiety-like responses elicited by unconditioned stimuli, such as bright light—that elicits anxiogenic behavior in rodents—and infusion of corticotrophin-releasing hormone (CRH)—that elicits anxiety-like responses similar to natural stressors. In later research it was discovered that, in contrast to amygdala lesions, BNST lesions abolished anxiety-like response to both bright light and CRH infusion (Lee and Davis, 1997; Walker and Davis, 1997); importantly, BNST lesions did not affect conditioned fear, demonstrating a double dissociation between the amygdala and BNST. This was an intriguing dissociation given the dense structural connections between the BNST and amygdala, strong cytoarchitectural and chemoarchitectural similarities between regions, and given that each region is integrated into a highly overlapping structural network (see BNST Structural Characteristics, below). However, it is important to keep in mind that the relationships between the amygdala and BNST, and between fear and anxiety, likely overlap and are inherently complex.
A series of elegant follow-up studies has further clarified the roles of the amygdala and BNST in the production of dissociable threat responses (see Davis et al, 2010; Walker et al, 2003, 2009 for review) and findings from other groups have also supported a unique role for the BNST (Sullivan et al, 2004; Waddell et al, 2006). Thus, mounting evidence now suggests that the amygdala mediates short-term, phasic responses to immediate threats, whereas the BNST mediates sustained responses to contextual, diffuse, and unpredictable threats. Recent studies have shown that the BNST also mediates hypervigilance and arousal (Davis et al, 2010), increased sensitization to the environment (Davis and Walker, 2013), and stress-enhanced learning (Bangasser and Shors, 2008), with recent optogenetics research further emphasizing the central role of the BNST in myriad stress and anxiety-related behaviors (Kim et al, 2013).
Of interest, anxiolytic pharmacological agents have distinct effects on BNST-mediated sustained responses relative to amygdala-mediated phasic responses. For example, Miles et al (2011) demonstrated that both benzodiazepine (chlordiazepoxide) and chronic administration of a serotonin selective reuptake inhibitor (SSRI; fluoxetine) significantly reduced sustained fear responses but had no effect on phasic fear responses. In contrast, the 5HT1A agonist (buspirone) reduced phasic but not sustained responses, highlighting a pharmacological dissociation. Benzodiazpine administration also reduced CRF-enhanced startle, suggesting that the effect generalizes to other paradigms that measure BNST-mediated responses (Swerdlow et al, 1986). Another study showed that an acute injection of fluoxetine into the BNST of fear conditioned rodents enhanced fear acquisition and this effect was not seen for injections into the amygdala (Ravinder et al, 2013). Together, these findings suggest that the BNST and BNST-mediated behaviors respond uniquely to pharmacological agents, although much remains to be learned about the exact nature of these relationships and the implications for clinical practice.
Neuroimaging studies in macaques provide confirmatory support for the role of BNST in anxiety behavior. In most of these studies, anxiety has been elicited using an averted eye gaze paradigm that signals potential social threat. The macaque BNST is activated when viewing an aggressive peer monkey face with an averted eye gaze (Hoffman et al, 2007) and BNST activity correlates with freezing duration in the presence of a human intruder with averted eye gaze (Kalin et al, 2005). Freezing is an adaptive response that allows monitoring of threat while avoiding detection, analogous to rodent anxiety-like behaviors that are prominent during the postencounter defensive stage. These studies provided initial evidence of cross-species similarities in BNST function and laid the groundwork for early studies in humans.
In line with findings in rodents and non-human primates, the National Institute of Mental Health Research Domain Criteria (RDoC) Negative Valence workgroup proposed separate construct definitions for response to acute threat (amygdala-mediated ‘fear’) and response to potential threat (BNST-mediated ‘anxiety’). Together, rodent findings and the RDoC construct of response to potential threat have sparked considerable interest in determining whether the BNST is a neural substrate of anxiety in humans.
AN EMERGING ROLE FOR THE BNST IN ADDICTION
Drugs of abuse produce changes in neural circuits mediating reward and stress. The BNST is a core component of the stress neural circuit and importantly has bidirectional connections with the ventral tegmental area (VTA)—a region centrally involved in addiction processes (Jennings et al, 2013b). For excellent, comprehensive reviews of BNST circuitry and addiction, see Stamatakis et al (2014, Kash (2012), and Koob and Volkow (2010).
Whereas early models of addiction focused on hedonic reward, recent research findings have emphasized the role of negative reinforcement in maintaining addictive behaviors, even in the absence of a pleasurable experience. In contrast to positive reinforcers that strengthen behavioral response through a pleasurable or rewarding stimulus, negative reinforcers strengthen behavior by removing negative stimuli. Thus, addictive behaviors may be maintained by allowing the individual to escape from the negative affect, anxiety, and dysphoria common during drug withdrawal. Koob and Volkow (2010) provide a comprehensive model of addiction neurobiology that proposes distinct neural circuits for three stages of addiction: binge/intoxication; withdrawal/negative affect; and preoccupation/craving addiction (also see Koob and Moal, 1997). In the Koob and Volkow model, the BNST has a prominent role in the withdrawal/negative affect stage of addiction, consistent with evidence that highlights the role of BNST in withdrawal across drugs of abuse, including alcohol (Silberman et al, 2013; Wills et al, 2012) and opiates (Wang et al, 2006; Delfs et al, 2000). The withdrawal/negative affect stage has been described as the ‘dark side of addiction’ and includes core components of anxiety disorders. At this stage, negative reinforcement likely drives craving and relapse. When considering the overlap between addiction and anxiety, it is interesting to note that for individuals with anxiety disorders negative reinforcement (not reward) may drive initial drug use, especially use of central nervous depressants such as alcohol and marijuana.
Although the role of BNST in withdrawal is highlighted in models of addiction neurocircuitry, evidence suggests that the BNST may also be involved in other stages of addiction. For example, the BNST is involved in the anxiogenic effects of cocaine intoxication (Wenzel et al, 2014). In addition, there is compelling evidence that the BNST mediates stress-induced reinstatement of drug-seeking behavior (Buffalari and See, 2011; Erb et al, 2001; Flavin and Winder, 2013; Jennings et al, 2013a), thus serving an important role in understanding relapse following a period of abstinence.
Similar to the light-enhanced startle and CRF-enhanced startle described earlier, withdrawal from drugs of abuse also enhances startle response, providing a model for testing effects of pharmacological agents on withdrawal. CRF and norepinephrine (NE) receptors in the BNST have been implicated in drug withdrawal-induced anxiety (see Aston-Jones and Kalivas, 2008; Logrip et al, 2011 for reviews), making these receptors prime targets for pharmacological interventions. Park and colleagues (2013) studied heroin withdrawal-potentiated startle and found that both CRF1 antagonist (MPZP) and α2 adrenergic receptor agonist (clonidine) block the withdrawal-potentiated startle. In another study, both clonidine and chlordiazepoxide blocked morphine withdrawal-potentiated startle (Harris and Gewirtz, 2004). These studies highlight the potential of pharmacological agents to modify BNST-mediated drug withdrawal.
In contrast to anxiety, the CeA and BNST are thought to play a similar role in addiction; therefore, most studies have not systematically tested for differences. However, there is some evidence that reinstatement of drug-seeking behavior is uniquely mediated by the BNST (Erb et al, 2001; Lu et al, 2003; Shaham et al, 2003), although many addiction studies find similarities in CeA and BNST response (eg, McFarland et al, 2004). These studies highlight the importance of the BNST for understanding mechanisms underlying addiction.
THE HUMAN BNST
To date, only a handful of studies have reported BNST activity in humans in contrast to the nearly 5,000 human imaging studies reporting on the amygdala. We believe that this small but growing field has tremendous capacity to significantly inform our understanding of the pathophysiology of two of the most prevalent and costly psychiatric disorders affecting children and adults—anxiety and addiction (Erskine et al, 2014; Kessler et al, 2005a, b; Whiteford et al, 2013). If alterations in BNST structure, function, or connectivity indeed underlie these disorders in humans, then the BNST becomes a novel, untapped treatment target. Supporting this potentially novel role is evidence from rodent studies that anxiety and addiction processes may be altered by drugs that target the BNST, including CRF antagonists (Walker et al, 2009), norepinephrine agonists (Park et al, 2013; Harris and Gewirtz, 2004) and antagonists (Wenzel et al, 2014), and endocannabinoid system targets (Glangetas et al, 2013).
BNST Structural Characteristics
The BNST is small structure located in the medial basal forebrain in humans. At ∼190 mm3, the human BNST is about the size of a small sunflower seed and ∼1/10th the size of the amygdala. Situated centrally in the brain, the BNST is located posterior to nucleus accumbens, anterior to thalamus, inferior to the lateral ventricles, and medial to the internal capsule and caudate (Figure 2). The BNST is situated mostly superior to the anterior commissure in humans, although the human BNST includes a ventrolateral continuity extending below the anterior commissure, as in rodents (Walter et al, 1991). The human BNST is typically subdivided along a medial–lateral organization (Walter et al, 1991), with medial, central, and lateral subdivisions showing correspondence with typical rodent subdivisions; humans may also show a differentiation between anterior and posterior BNST subnuclei (although see Walter et al, 1991 for comparison with rodent subdivisions).
The human bed nucleus of the stria terminalis (BNST). (Left) The human brain is shown as an illustration with the BNST highlighted in yellow (Gray, 1918). (Middle) For reference, a similar slice is shown for fixed tissue (Mai et al, 2008). (Right) Our BNST mask is outlined in yellow on a 7T gradient spin echo (GRASE) magnetic resonance image (adapted from Avery et al, 2014). Note that in the 7T mask we use the anterior commissure as the ventral boundary of the BNST—the subcommissural extent of the BNST is difficult to distinguish with certainty from surrounding tissue because of MRI partial volume effects and its small size.
The BNST is part of the ‘extended amygdala’—a set of brain regions often referred to as a single entity based on their similar ontogeny, cytoarchitecture, chemoreceptors, and structural connections (Alheid and Heimer, 1988; Heimer and Alheid, 1991)—along with the central and medial amygdala subnuclei, and a transition zone in the medial (shell) subregion of the nucleus accumbens. In non-human primates, the extended amygdala is structurally connected via two major white matter tracts: the most prominent is the stria terminalis (Price and Amaral, 1981), a white matter bundle that extends superiorly and anteriorly from the corticomedial amygdala, wraps around the thalamus in a C-shape similar to the fornix, and descends to the BNST with some fibers continuing rostrally to the accumbens; the second is the less studied but more direct connection formed by the ventral amygdalofugal pathway, a diffuse collection of fibers that extends from the basolateral and central amygdala dorsally to the BNST (Porrino et al, 1981).
Translating Rodent Anxiety to Humans
Anxiety is a future-oriented state elicited by threats that are physically distant, psychologically distant, or unpredictable; for example, in humans, an upcoming performance evaluation or a talk in front of colleagues is effective in eliciting anxiety. Fear, on the other hand, is a phasic state of heightened arousal and orienting toward an immediate and identifiable danger, such as the nearby screeching of car brakes. Although anxiety and fear are overlapping in nature, there is general acceptance among clinicians for a dissociation between the worry and tension of anxiety and the short-lived, intense fear response (Brown et al, 1998; Zinbarg and Barlow, 1996). A similar contrast can be found in studies of defensive behavior in rodents. In rodents, anxiety-like behaviors are elicited by physically distant threats, such as a physically distant predator, or diffuse contextual threats, such as a location previously paired with a painful footshock. In contrast, fear-like behavior is elicited by more physically proximal or imminent threats. This distinction between distant and proximal threat provides a useful perspective for designing and interpreting human threat studies. To translate this idea to humans, we propose that anxiety is more often elicited by psychologically distant rather than physically distant threats. Consistent with this idea, human investigations of anxiety have largely used psychologically distant threats, such as anticipation of an unpredictable threat; these paradigms are analogous to diffuse threat paradigms that elicit rodent anxiety-like behavior (Figure 3). Thus, human anticipation tasks provide an optimal translational tool for exploring evolutionarily conserved neural and physiological responses to potential threat. Here we review evidence of BNST engagement in humans (Table 1). We also explore whether the central differentiation of rodent fear and anxiety research—the functional dissociation between the amygdala and BNST—exists in humans.
A model for anxiety and fear in rodents and humans. There are similarities between the defensive distance continuum demonstrated in rodents and the analogous predictable threat continuum in humans. Stages of threat can vary along either physical or psychological spectra ranging from distant–close physical threat and unpredictable–predictable psychological threat. In rodents, distant threats elicit anxiety-like behaviors mediated by the BNST; as threats move closer, amygdala-mediated fear behaviors become prominent. In humans, anxiety is elicited by unpredictable threat tasks; anticipation of unpredictable threat engages the BNST; in contrast, predictable threat tasks engage the amygdala. These findings in humans parallel the neural dissociation observed in rodents, suggesting a parallel model in rodents and humans: threats that are spatially and temporally distant provoke anxiety and are mediated by the BNST, whereas threats that are spatially or temporally close provoke fear and are mediated by the amygdala.
BNST Function during Anticipation of Threat
Paradigms using shock are often used in rodent studies to elicit phasic fear (‘fear’) and sustained fear (‘anxiety’) responses. Therefore, the most direct translation from rodent paradigms to human research is anticipation of shock tasks that have been used in several studies. As one example, the NPU-threat task (no-shock/predictable-shock/unpredictable-shock task) developed by the Grillon lab (Schmitz and Grillon, 2012) manipulates threat context to elicit safety, fear, and anxiety responses, respectively. In the no-shock/safe condition, no shocks are administered in a particular context, providing a baseline measure. In the predictable-shock condition, participants are trained that within a particular context, a cue (eg, a tone) is reliably paired with an electrical shock. The tone is meant to elicit a fear response. In the unpredictable threat context, electrical shocks could be administered at any time. In this context, the cue does not signal the timing of the shock; rather, the shock is unsignaled to create a sense of anxious apprehension. Participants are explicitly told which contexts are associated with each condition, minimizing individual differences in learning.
Unpredictable threats associated with anxiety-like behaviors have been linked with the BNST in rodents—does unpredictable threat elicit BNST activity in humans? Using the NPU-threat task, Alvarez et al (2011) examined BNST activity during 40 s unpredictable threat contexts in humans. Consistent with findings in rodents (Walker et al, 2003), the BNST showed the greatest response in the unpredictable threat context followed by predictable threat and no threat contexts. Skin conductance response—a measure of arousal—and self-report anxiety ratings paralleled BNST findings (Alvarez et al, 2011). Together, these findings provided strong initial evidence that the BNST tracks anxiety elicited by threat in humans.
Does the BNST respond selectively during long-duration contexts in humans? Findings from rodent studies have highlighted that the BNST responds to long-duration contexts, whereas the amygdala responds to short-term cues. The previously described study (Alvarez et al, 2011) confirmed that long-duration contexts can engage the BNST; however, two other studies have shown BNST activity in response to much shorter anticipation periods. Choi et al (2012) found elevated BNST activity and skin conductance response during a short 1.75–5.75 s threat context. In another study, Klumpers et al (2014) found greater BNST activity to threat (relative to safe cues) using a 4-s shock anticipation period. McMenamin et al (2014) explicitly tested the time course of BNST by examining BNST response during early (~0–10 s), middle (~10–20 s), and late (~20–40 s) phases of a 40-s cued anticipation period. The BNST showed elevated activity beginning in the middle time period, with a trend (P=0.08) during the late time period, consistent with a sustained hypervigilance response. Thus, these studies suggest that both short-term cues and long-duration contexts are sufficient to engage the BNST; however, future studies are needed to systematically examine this question.
The above studies demonstrate that the BNST responds to threat of imminent shock, but does the BNST also mediate worry about future events, a key component of human anxiety? To examine this question, Somerville et al (2010) measured brain activity and skin conductance response during a threat anticipation task where participants were told they were earning shocks to be administered at the end of the task, as a measure of worry about future events. Possibility of future shock was varied across three levels: low risk, medium risk, and high risk. BNST activity tracked with shock risk and was greatest during the high-risk periods. In addition, BNST activation and skin conductance increased throughout the task, suggesting that closer temporal proximity of the threat resulted in stronger anxiety. These findings suggest that the BNST is engaged by worry about future events but also tracks temporal proximity of threat, showing highest activity when a threat is on the verge of occurring.
Does the BNST also monitor spatial proximity of threat? Heightened vigilance to threats that are nearby, relative to far away, is evolutionarily adaptive for both rodents and humans as it promotes activation of the stress response system and downstream pathways, increasing the ability to respond or avoid the threat quickly if it moves close (Bolles and Fanselow, 1980). In rodents, defensive patterns vary with physical distance from a potential threat (Blanchard et al, 1993; Fanselow, 1986), with hypervigilant behaviors becoming more prominent when threats are proximal but not immediate. Mobbs et al (2010) examined the effect of proximity to threat by showing participants a video of a live tarantula moving freely in a compartmented box attached to the participant’s foot. The tarantula was randomly placed into compartments at varying distances from the foot. BNST activation and self-reported anxiety increased as the tarantula moved closer to the foot and, importantly, also decreased as the tarantula moved further away. Thus, the human BNST is sensitive to escalation of threat by tracking not only temporal proximity, but also increasing spatial proximity.
Would the BNST respond to less salient threats, such as aversive images? The majority of studies reporting BNST activity in humans have used an intense physically aversive stimulus—shock. However, most studies of emotion processing and anxiety in humans have used aversive images. To determine whether the BNST responds to anticipation of viewing an aversive image, Grupe et al (2013) replaced shock with aversive images (IAPS) and measured sustained anticipation over a 2–8 s cued threat period. Relative to neutral image anticipation, the BNST showed a sustained elevated response during anticipation of aversive images. However, many studies examining anticipation of viewing aversive images have not reported BNST activation. Given the very small size of BNST and corresponding technical difficulties using standard neuroimaging methods, it is possible that the activity of the BNST was simply undetected in early studies. These results indicate that the BNST is not only sensitive to threat of physical harm (shock), but is also engaged by emotional stimuli.
The BNST is engaged during active anticipation of threat—does it also play a role in imagining potential threats? Coaster et al (2011) asked participants to evaluate the risk of scenarios with either high or low harm potential (eg, death, a stubbed toe). When evaluating high compared with low harm scenarios, BNST activity was elevated, suggesting that the BNST is engaged when imagining potential threat, particularly when those threats are serious.
One of the central findings from rodent BNST research is the dissociation between the role of the BNST in anxiety and the CeA in fear—is there also evidence of dissociable roles in humans? Alvarez et al (2011) used the NPU-threat task to examine brain responses during unpredictable contexts compared with predictable contexts. Analogous to findings in rodents, the BNST was active during unpredictable threat (‘anxiety’) conditions but not during predictable threat (‘fear’) conditions, whereas the amygdala showed the opposite pattern—the amygdala was active during predictable threat conditions but not unpredictable threat conditions—suggesting a neural dissociation in humans similar to rodents. Consistent evidence is found in studies that compared unpredictable with safe contexts and report no evidence of amygdala activity (Choi et al, 2012; Grupe et al, 2013; Klumpers et al, 2014; McMenamin et al, 2014). In addition, although Somerville et al (2010) found increased amygdala ROI activity in a simple contrast of threat>safe, amygdala activity did not show a linear increase with increased risk of shock, was not significant in the whole-brain analysis, and did not show a relationship with trait anxiety—findings that were all observed for the BNST. Together, these results support the notion of a dissociation between the BNST and amygdala in humans in response to different types of threat; however, it is not surprising that there are also overlaps in function between the BNST and the amygdala, given strong reciprocal connections between regions. For example, Mobbs et al (2010) found increased amygdala activity with increasing spatial proximity of threat (tarantula), as well as increased activity during approach relative to withdrawal of the threat, findings that are qualitatively similar to the BNST findings reported in the same study. Extensive interactions between the BNST and amygdala are likely necessary as threats move closer and defensive behaviors must shift from BNST-mediated anxious hypervigilance to CeA-mediated fear (Hitchcock and Davis, 1986, 1987). However, Mobbs et al (2010) also reported a dissociation between amygdala and BNST response—the amygdala showed significant habituation across the course of the tarantula experiment whereas the BNST did not, supporting rodent and human findings of a phasic role for the amygdala in response to persistent threat and a sustained role for the BNST. Together, these initial findings provide preliminary support for the idea that the BNST is engaged by distal threat and anxiety. However, both Alvarez et al (2011) and Mobbs et al (2010) also show amygdala activation during threat conditions, highlighting the well-documented role of the amygdala in evaluating threat; therefore, future studies are needed to directly test for a dissociation between the BNST and amygdala using a variety of tasks designed to probe proximal and distant threats.
The Challenge of Human BNST Research
The findings reviewed above show that the human BNST responds to anticipation of an upcoming aversive event, and this BNST response generalizes across multiple different anticipation tasks and different degrees of threat. However, more precise investigation of the BNST is needed to fully uncover its role in threat anticipation and human anxiety. The primary obstacle to studying the BNST using in vivo imaging in humans has been a combination of the very small size of the BNST along with the relatively low resolution of functional magnetic resonance imaging (fMRI). The BNST is an extremely small structure that is difficult to resolve from surrounding anatomy at standard spatial resolutions and signal-to-noise strength (3T) in neuroimaging data. Consistent with this obstacle, most previous studies have interpreted their findings cautiously as ‘overlapping with a region consistent with the BNST.’ To overcome these challenges and facilitate human neuroimaging studies of the BNST, we collected ultra-high-resolution data at 7T using a gradient spin echo (GRASE) sequence specifically designed to optimally resolve the BNST in contrast to nearby structures by creating clear contrast between gray matter, white matter, and cerebrospinal fluid (Avery et al, 2014). With the aid of this mask, we were able to map the structural and functional connectivity of the BNST in humans for the first time (reviewed below).
To help clarify the spatial locations of the findings reported in the studies reviewed above, we performed a meta-analysis of the studies that used healthy control participants (selected studies are indicated with an asterisk in Table 1). Briefly, a GingerALE (version 2.3.2) random-effects analysis (Eickhoff et al, 2009) was performed using peak voxel coordinates from threat>safe contrasts with smoothing kernel 8.5–9.5 mm FWHM. The meta-analysis included all of the significant peaks reported in those studies across the brain, including the peaks identified as BNST. The resulting activation likelihood map was thresholded at P<0.001 to illustrate the brain regions showing significant likelihood of activation in threat>safe contexts (Figure 4). To determine the extent to which these findings included the BNST, we overlaid our anatomical mask generated from our 7T MRI data (also shown in Figure 4) on the activation likelihood map. This comparison revealed significant activation in the right anterior BNST, consistent with our BNST mask. In the left hemisphere, the strongest activation peak was in the head of the caudate with additional activation just lateral of the left BNST; however, it should be noted that at a more lenient threshold activation was also observed in the left BNST. Although this meta-analysis provides a useful summary of the neuroimaging findings from the studies reviewed here, it should be noted that the sample of studies were selected based on a reported BNST finding and therefore the observed P-values may not generalize to a sample of all studies of anticipation of threat.
Meta-analysis of BNST findings in human threat anticipation studies. We performed a GingerALE meta-analysis of the reported BNST coordinates from nine human imaging studies, thresholded at P<0.001 (top); included studies are marked with an asterisk in Table 1. Our BNST mask overlays the meta-analysis (bottom). The color map represents activation likelihood values.
THE BNST NEURAL NETWORK
BNST Connectivity in Rodents and Non-Human Primates
Tracer studies in rodents have characterized the extensive structural connections of BNST to other limbic regions—including the amygdala, hypothalamus, hippocampus, periaqueductal gray, infralimbic cortex (part of the subgenual cingulate/ventromedial prefrontal cortex), and anterior insula (Dong et al, 2001; Dong and Swanson, 2004a, b, 2006a, b, c)—and striatal regions, including the nucleus accumbens and VTA (Dong et al, 2001). Together, the limbic connections of BNST form a BNST-mediated anxiety circuit (Table 2 and Figure 1). Interactions between the BNST and other parts of the anxiety circuit are likely controlled through a feedback inhibition system, and lack of inhibition may result in anxiety (Adhikari, 2014; Hammack et al, 2009). The BNST is also a key node in the addiction circuit mediating the negative affect stage of withdrawal; the BNST, striatum, and amygdala are proposed to underlie the negative emotion state that can incite relapse even after long periods of abstinence (Kash, 2012; Stamatakis et al, 2014). Non-human primate studies provide preliminary evidence for functional connectivity in an anxiety circuit with strong connectivity between the BNST and CeA during conditions of threat (Birn et al, 2014) and at rest (Oler et al, 2012). There is also preliminary evidence that the amygdala, hippocampus, and periaqueductal gray together demonstrate a trait-like pattern of brain activity across multiple experimental conditions in macaques, and that this trait-like activity across regions is predictive of individual differences in temperament, a risk factor for anxiety disorders (Fox et al, 2008). Together, these studies provide evidence for patterns of BNST structural and functional connectivity that lay the groundwork for human studies.
BNST Structural Connections in Humans
The BNST is larger (proportional to brain size) and more developed in humans than in rodents (Lesur et al, 1989), suggesting that its structural connections may differ. Tract tracing studies in rodents have shown that the BNST is situated at the intersection of key circuits central to threat and salience processing, learning and memory, and motivation and reward (Table 2). We investigated BNST structural connectivity within these circuits for the first time in humans using a novel diffusion tractography analysis (Avery et al, 2014). To delineate human BNST structural connections across the whole brain, we employed a two-step analysis: we first performed a bootstrapping analysis to identify significant connections to a set of Harvard-Oxford atlas regions (Desikan et al, 2006) based on diffusion connectivity strength; then, within each identified region, we mapped the spatial pattern of BNST connectivity. This analysis revealed the human BNST was structurally connected with a number of limbic, thalamic, and basal ganglia structures, confirming known rodent connections for the first time (Figure 5). Our analysis also described a connection within a frontotemporal transitional zone near the temporal pole, centered on the limen insulae. Although the location of this connection is novel in humans, the limen insulae is comprised of agranular neurons homologous to the caudal agranular insula in rodents that shows strong reciprocal connections with the BNST (Ding et al, 2009; Reynolds and Zahm, 2005; Turner and Zimmer, 1984), suggesting that this is an evolutionarily conserved connection across species. A recent study replicated a similar structural connection between the BNST and temporal pole region in humans (Krüger et al, 2015). The post hoc analysis of the anterior and posterior insula, separately, revealed a significant connection with the anterior insula as well. These findings confirm that humans and rodents share broad structural similarities in BNST connectivity, although the vast expansion of the human cortex may shift the spatial distribution of connections.
Structural and functional connectivity of the human BNST. (a) Our structural connectivity analysis reveals anatomical connections between the BNST and multiple limbic and striatal structures, largely confirming anatomical connections in rodents (Avery et al, 2014). (b) Our resting-state functional connectivity analysis reveals a network of connections mostly overlapping with BNST anatomical connections, with the addition of a novel prefrontal cortex connection (Avery et al, 2014). Functional connections are not constrained by direct structural connections, instead providing evidence of the broader network of regions involved in accomplishing a task. (c) Importantly, the BNST functional connectivity network has been replicated in a recent study (McMenamin et al, 2014).
BNST Functional Connections in Humans
How does the BNST functionally interact with its surrounding network? Resting-state functional connectivity, which measures synchronized fluctuations in brain activity between brain regions at rest, provides an important window into the intrinsic organization of the brain. Using this method, Oler et al (2012) demonstrated functional interactions between the BNST and CeA in humans, consistent with the notion that these distinct but highly interconnected regions have important continuous interactions in the human brain. However, although this study provided important initial evidence for BNST functional connectivity in humans, the full complement of regions intrinsically connected with the BNST remained unknown.
To characterize the functional connectivity of BNST throughout the brain, we measured resting-state functional connectivity across the brain using our anatomically defined BNST mask (Avery et al, 2014). Similar to our structural connectivity analysis (described above), we used a two-step method to identify and map individual regions with the strongest evidence of a functional connection. This analysis revealed resting-state functional connections between the BNST and multiple subcortical regions, including limbic, thalamic, and basal ganglia structures (Figure 5). These findings overlapped extensively with our structural connectivity findings and with known rodent connections, providing evidence of ongoing functional crosstalk, even at rest, among this structurally connected network of regions. Our analysis also revealed a novel functional connection between the BNST and paracingulate gyrus, part of the prefrontal cortex, in humans. This finding is especially interesting given the role of prefrontal cortex deficits in stress-related disorders such as anxiety and addiction (Koob and Volkow, 2010; Shin and Liberzon, 2010). Although no known structural connections exist between the BNST and paracingulate gyrus, functional connectivity is not constrained by monosynaptic structural connections, and BNST–prefrontal cortex interactions may be mediated through a third region. A recent study has since replicated this functional connectivity network (McMenamin et al, 2014), indicating that functional BNST connectivity with these regions is reliable across samples and laboratories (Figure 5). Overall, these findings define for the first time a set of BNST connections in humans that may form the core for anxiety and addictive disorders, laying the groundwork for future studies in psychiatric disorders.
What brain regions drive BNST function? Lesion studies, although rare in humans, can provide valuable causative information about the role of a region in a particular function. One such study has provided insight into functional interactions of the BNST in humans. Motzkin et al (2014) examined the effect of lesions in the ventromedial prefrontal cortex (vmPFC)—a region functionally connected with the BNST—on BNST activity. The vmPFC plays an important role in regulating emotions by providing top-down inhibition of brain regions involved in negative affect processing, such as the amygdala; however, the vmPFC also has strong structural connections with the BNST via the subgenual cingulate (Avery et al, 2014). Compared with healthy controls, patients with vmPFC lesions had significantly lower activity in the right BNST at rest. These findings suggest a driving role for the vmPFC over BNST activity in healthy adults, and provide the first causative association with the BNST in humans.
Whereas resting-state functional connectivity measures intrinsic correlations in activity across networks, task-based functional connectivity measures how activity in networks changes with a task. Task-based connectivity analyses can provide valuable insights into how brain regions interact within their network to process information. The Pessoa lab has examined threat-related changes in BNST connectivity in two recent studies (Kinnison et al, 2012; McMenamin et al, 2014). In the first study, Kinnison et al (2012) showed increased BNST functional connectivity with both the anterior insula and dorsomedial PFC during threat of shock. The anterior insula and dorsomedial PFC have known functional roles in threat processing and anxiety responses and are prominent members of the neural salience network (Downar et al, 2002; Eickhoff et al, 2014); these regions likely interact with the BNST to express an aversive emotional state during anticipation of threat (Drabant et al, 2011; Klumpers et al, 2014; Mechias et al, 2010; Riga et al, 2014). In a second study, McMenamin et al (2014) showed that the BNST had more functional interactions with its surrounding network during threat of shock, but only for the most anxious participants. This finding contributes further evidence that alterations in increased BNST activity may not only underlie current anxiety state, but may also play a role in chronic, trait anxiety. Although the participants included in the studies discussed thus far have had normative levels of trait anxiety, we review evidence below of altered BNST function in maladaptive anxiety.
Summary of Human BNST Connectivity
These findings show that the human BNST neural network comprises structural and functional connections with a number of limbic, striatal, and cortical regions. Human structural connections largely mirror rodent structural connections, although the vast expansion and elaboration of structures in the human brain mandates further research of the precise spatial mapping of connections in humans. In addition, only a subset of structural connections has thus far been investigated in humans (Table 2); therefore, further investigation of the BNST structural network is necessary to fully define the human BNST structural network. Functional connectivity studies show intrinsic interactions between the BNST and a broad network of regions not completely overlapping with structural connections, suggesting that BNST influence is wide-reaching. This notion is in line with the critical role that BNST plays in survival—like an orchestra conductor, the BNST must continually receive, process, and convey information about potential environmental threats to a wide network of regions. Network-based findings show that the role of BNST as conductor becomes more prominent during times of threat, suggesting that the BNST may be central to coordinating broad-scale threat reactivity across a multitude of regions that are involved in emotional and physiological preparedness. We propose that when coordinated functional activity breaks down, then stress-related disorders such as anxiety and addiction result. However, this question has yet to be addressed systematically—the reviewed structural and functional connectivity studies investigated healthy individuals. Below, we review evidence that BNST function is altered in anxiety and addiction.
STRESS-RELATED DISORDERS: EVIDENCE OF BNST DYSFUNCTION IN ANXIETY DISORDERS AND ADDICTION
Stress is a potent catalyst for structural and functional changes in the brain and is hypothesized to play a prominent role in the etiology of anxiety and addiction disorders. Given the prominent role of BNST in both anxiety and HPA activation, the BNST may be a key region affected by stress. Early-life stressors are thought to play an especially important role in risk for later anxiety and addiction disorders, although the role of the BNST in this process remains largely unknown. Banihashemi et al (2014) examined the effect of a history of maltreatment on BNST activity during stress. Brain activity and physiological response (mean arterial pressure) was measured during a mental stress task. Degree of childhood physical abuse was correlated with both stress-related increase in mean arterial pressure and decreased activity in the BNST. Changes in limbic activity have been proposed to be a marker of stress response, and limbic activity is proportional in size to cortisol release during stress (Pruessner et al, 2008). This initial study suggests that early stressor may alter later BNST function; however, future studies are needed to further clarify the role of trauma in BNST function.
BNST Function in Anxiety Disorders
Low-level anxiety is an evolutionarily adaptive survival response (Ohman, 1986); the careful monitoring of a potential threat, along with corresponding physiological arousal, primes the ‘fight or flight’ system for a response should the threat become imminent. For our ancestors, hypervigilance to a rustling noise in the tall grass nearby was critical—it may have meant the difference between being eaten by a lion and escaping unscathed. Anxiety remains an important tool for survival even in modern times; low-level anxiety has been shown to benefit preparation and performance for stressful events (eg, public speaking) and can help us avoid potentially dangerous situations (Jamieson et al, 2013; Lupien and Mcewen, 1997; Price, 2003). In contrast, high-level anxiety hinders performance and is a maladaptive response (Lupien and Mcewen, 1997). Indeed, chronic, high-level anxiety is considered pathological and forms the core of anxiety disorders.
We have reviewed evidence that the BNST is engaged by anxiogenic tasks in healthy adults, with two studies showing individual differences in normative anxiety are associated with greater BNST activation (Somerville et al, 2010) and functional connectivity (McMenamin et al, 2014)—a key question is whether altered BNST engagement is a marker of maladaptive anxiety. Straube et al (2007) examined BNST function in spider phobics using a cued-threat task where a cue indicated whether a threat (spider) or control (mushroom) image would be presented. Spider phobics showed greater BNST activity during anticipation of spider images relative to the mushroom image. In a group of generalized anxiety disorder (GAD) patients, however, Yassa et al (2012) failed to show a statistically significant difference in BNST response to high vs low stress conditions relative to controls, although GAD patients showed marginally greater BNST activity (P=0.10). Task differences between these two studies may be key—Yassa et al (2012) manipulated stress using a monetary loss task that may promote a very different brain response than threats such as shock or spiders. For example, a task examining monetary loss in healthy controls found only phasic BNST activity (Schlund et al, 2013), suggesting that potential loss of reward does not strongly engage the BNST. Thus, further studies are needed to disambiguate the role of the BNST in anxiety disorders in humans.
Taken together, however, these studies hint that elevated BNST activity may be a useful marker of maladaptive anxiety. Intriguingly, both studies failed to find elevated amygdala response, often considered a hallmark neural signature of anxiety. Further studies are necessary to understand potentially complicated BNST–amygdala interactions, specifically how interactions between these regions contribute to anxiety disorders. In light of the evidence reviewed thus far, we propose that future studies of anxiety should take steps to specifically investigate the BNST as a critical node in the anxiety network.
BNST Function in Addiction Disorders
Two studies have supported a role for the BNST in addiction in humans. In a study of nicotine-addicted participants, Dagher et al (2009) showed that a psychosocial stressor task elicited widespread changes in limbic (including BNST) activity—a marker of stress response (Pruessner et al, 2008)—and that changes in limbic activity were predictive of subsequent increased BNST activity to smoking cues. In a study of alcohol addiction, O’Daly et al (2012) demonstrated that patients with an alcohol use disorder showed increased functional connectivity between the BNST and amygdala while viewing fearful faces, relative to controls. Together, these studies show initial support for a role for the BNST in addiction processes.
SUMMARY AND CONCLUSIONS
Rodent models of fear, anxiety, and addiction have highlighted the importance of a tiny brain region—the BNST—in the development and maintenance of these illnesses. Although methodological and technological limitations prevented earlier investigation of the BNST in humans, we and others have been able to overcome these obstacles, setting the stage for future studies. Indeed, during the past 4 years, we have seen an exponential growth of human imaging studies of the BNST. Although the field is still in its infancy, several common findings are emerging: (1) the BNST is engaged during anticipation of an unpredictable threat, analogous to findings in rodents; (2) the BNST is activated both in longer duration contexts (~1 min) and in response to brief cues; thus, whereas the BNST mediates responses to sustained cue in rodents, human studies show that even very brief cues are sufficient to engage in the BNST; (3) preliminary evidence suggests that the BNST may be preferentially activated during anticipation of an aversive event, although future research is needed directly comparing BNST with amygdala responses; (4) both strong threats, like shocks, and more mild threats, like aversive images, engage the BNST; this finding is especially important for extending these tasks to populations where shock is not possible; (5) although human anxiety is often focused on psychological or unpredictable threats, the BNST is also engaged by increasing spatial proximity of threat; there is also evidence that the amygdala is similarly engaged by spatial proximity of threat, suggesting an important interaction between the two regions that may underlie the transition from BNST-mediated anxiety behaviors when threats are distant to amygdala-mediated fear behavior as threats become close; (6) BNST structural connectivity largely maps onto findings in rodents and non-human primates, with the exception of the prefrontal cortex findings, and this is not surprising given the vast expansion of the prefrontal cortex in humans; and (7) BNST functional connectivity has been established and replicated in an independent sample, providing a guide for future studies of task-based connectivity. Ultimately, investigation of the BNST in the human brain will continue to produce novel insights into the fundamental role of BNST in human stress-related psychopathology and will provide exciting opportunities for drug and therapeutic development.
FUTURE DIRECTIONS
Anxiety and addiction represent two of the most prevalent and costly stress-related illnesses, and initial evidence suggests a potential role for the BNST in both disorders. The studies reviewed here provide preliminary evidence that the human BNST is involved in anxiety—a critical translation of seminal rodent research—and serve as a springboard for important questions that still need to be asked in both humans and rodents. These are our suggestions for future research directions.
Test for Differences in BNST Structure, Function, and Connectivity in Patients with Anxiety and Addiction Disorders
Neuroimaging studies in other disorders, such as depression, illustrate how disrupted functional connectivity may underlie psychiatric disorders and how treatments that alter connectivity, such as deep brain stimulation, can ameliorate symptoms (Smart et al, 2015). Anxiety and addiction both stem from evolutionarily adaptive systems (fear and reward) that at some point go awry, producing maladaptive behaviors that endanger survival. Human studies of BNST function have been instrumental in clarifying the nature of normative BNST function in anxiety tasks; however, very few have examined BNST function in patients with anxiety or addiction disorders. Thus, a crucial next step in our field is to determine whether BNST function is altered in anxiety or addiction and, if so, to characterize the nature of the dysfunction. For example, in anxiety disorders the BNST may show hyperactive responses to potential threat or may show attenuated responses, secondary to chronic activation. In addition, treatment studies examining the effects of different treatments on the BNST may shed light into the role of BNST in treatment and recovery as well as individual differences in treatment response.
Examine BNST Function Across Development
A key feature of both anxiety and addiction is their developmental profile; both disorders have an early onset, typically by early adolescence (Erskine et al, 2014; Kessler et al, 2005a). Therefore, it is critical for future studies to investigate the BNST early in development. We expect the BNST to be largely mature by middle childhood, similar to the amygdala, but with continued room for development. As a preliminary step, we hope to see a mapping of the BNST structural and functional connectivity across multiple stages of development to identify potentially critical periods where networks change. Then, studies of young children at high risk for developing anxiety or addiction disorders will be important for determining whether BNST function is altered in these children.
Identify Novel Pharmacological Treatments
One of the reasons that we are most excited about exploring human BNST function is that the BNST represents a novel target for pharmacological treatments. Studies in rodents highlight the dissociation in pharmacological responses between phasic and sustained responses in rodents. Initial attempts to validate these findings in humans have been encouraging; for example, Grillon et al (2006) showed that benzodiazepine administration (alprazolam) reduced unpredictable context-enhanced startle but not fear-potentiated startle. In another study from the same lab (Robinson et al, 2012), reduced serotonin via acute tryptophan depletion enhanced startle in the long-duration threat periods but had no effect in the short-duration threat periods. Although more research is needed in this area, the preliminary results in both rodents and humans are encouraging. We are especially cheered by the number of pharmacological agents that specifically target BNST-mediated anxiety or withdrawal, including CRH antagonists, SSRIs, and α2 receptor agonists (eg, guanfacine). Research on these substances in animal models is active and there is hope that phase I clinical trials in humans will follow soon.
Characterize the BNST Neurocircuitry Underlying Stress-Related Disorders in Humans
Brain regions do not operate in isolation, especially when governing complex behaviors; therefore, it is critical to identify the core circuit underlying stress-related disorders in humans. Several studies have begun to map these circuits in healthy adults, providing an initial map for future investigations. Anxiety and addiction have excellent animal models and translational tasks—NPU, resting-state fMRI, and pharmaco-fMRI—that provide an exciting platform for rapid, back-and-forth translation from rodents to humans and back. Rodent findings have driven current human investigations and once core circuits are discovered in humans, these circuits can be systematically dissected and tested in rodents using lesion and optogenetic methods.
Advance Neuroimaging Technology to Facilitate Studies of the BNST
To continue to move the field forward, advances in neuroimaging technology and methods are needed. We used ultra-high field (7T) structural images to increase precise spatial localization of the BNST (Avery et al, 2014). Ultra-high field strength is ideal for structural studies of the BNST, although ultimately 7T functional MRI and DTI images are needed to provide more precise characterization of the structural and functional networks in humans. A remaining limitation in 7T fMRI is susceptibility to artifacts in key temporal lobe regions, including the amygdala, caused by the close vicinity to the sphenoid sinus and B0 and B1 inhomogeneity; thus, functional studies examining the whole brain remain difficult at ultra-high field strength. As methods advance, human studies will gain the precision needed to investigate functional differences in BNST subnuclei, providing an opportunity for translation back to rodents.
References
Adhikari A (2014). Distributed circuits underlying anxiety. Front Behav Neurosci 8: 112.
Alheid GF, Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27: 1–39.
Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage 55: 389–400 This study used a translational threat task to demonstrate that the human BNST is engaged during contexts of unpredictable relative to predictable threat, consistent with previous findings in rodents..
Aston-Jones G, Kalivas PW (2008). Brain norepinephrine rediscovered in addiction research. Biol Psychiatry 63: 1005–1006.
Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU (2014). BNST neurocircuitry in humans. Neuroimage 91: 311–323 This is the first study to characterize BNST structural and functional connectivity in humans and provide evidence of relative homology across species.
Bangasser DA, Shors TJ (2008). The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J Neurosci 28: 6383–6387.
Banihashemi L, Sheu LK, Midei AJ, Gianaros PJ (2014). Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Soc Cogn Affect Neurosci 10: 474–485.
Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM et al (2014). Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry 19: 915–922.
Blanchard RJ, Yudko EB, Rodgers RJ, Blanchard DC (1993). Defense system psychopharmacology: an ethological approach to the pharmacology of fear and anxiety. Behav Brain Res 58: 155–165.
Bolles RC, Fanselow MS (1980). A perceptual-defensive-recuperative model of fear and pain. Behav Brain Sci 3: 315.
Brown TA, Chorpita BF, Barlow DH (1998). Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol 107: 179–192.
Buffalari DM, See RE (2011). Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 213: 19–27.
Choi JM, Padmala S, Pessoa L (2012). Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage 59: 1912–1923.
Coaster M, Rogers BP, Jones OD, Viscusi WK, Merkle KL, Zald DH et al (2011). Variables influencing the neural correlates of perceived risk of physical harm. Cogn Affect Behav Neurosci 11: 494–507.
Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D (2009). An acute psychosocial stress enhances the neural response to smoking cues. Brain Res 1293: 40–48.
Davis M, Falls WA, Campeau S, Kim M (1993). Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 58: 175–198.
Davis M, Walker DL (2013). Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct Funct 219: 1969–1982.
Davis M, Walker DL, Miles L, Grillon C (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35: 105–135 Classic review paper highlighting the rodent literature on the dissociation between amygdala and BNST in fear vs anxiety.
Delfs JM, Zhu Y, Druhan JP, Aston-Jones G (2000). Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403: 430–434.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980.
Ding SL, Hoesen GW, Van, Cassell MD, Poremba A (2009). Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol 514: 595–623.
Dong H-W, Petrovich GD, Watts AG, Swanson LW (2001). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436: 430–455.
Dong H-W, Swanson LW (2004a). Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol 471: 396–433.
Dong H-W, Swanson LW (2004b). Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol 468: 277–298.
Dong H-W, Swanson LW (2006a). Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol 494: 142–178.
Dong H-W, Swanson LW (2006b). Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol 494: 75–107.
Dong H-W, Swanson LW (2006c). Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol 494: 108–141.
Downar J, Crawley AP, Mikulis DJ, Davis KD (2002). A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87: 615–620.
Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR et al (2011). Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage 55: 401–410.
Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L (2014). Functional segregation of the human dorsomedial prefrontal cortex. Cereb Cortex. (e-pub ahead of print; doi:10.1093/cercor/bhu250).
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30: 2907–2926.
Erb S, Shaham Y, Stewart J (2001). Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress 4: 289–303.
Erskine HE, Moffitt TE, Copeland WE, Costello EJ, Ferrari AJ, Patton G et al (2014). A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol Med 45: 1551–1563.
Fanselow MS (1986). Associative vs topographical accounts of the immediate shock-freezing deficit in rats: implications for the response selection rules governing species-specific defensive reactions. Learn Motiv 17: 16–39.
Flavin SA, Winder DG (2013). Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology 70: 324–330.
Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH (2008). Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One 3: e2570.
Glangetas C, Girard D, Groc L, Marsicano G, Chaouloff F, Georges F (2013). Stress switches cannabinoid type-1 (CB1) receptor-dependent plasticity from LTD to LTP in the bed nucleus of the stria terminalis. J Neurosci 33: 19657–19663.
Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W et al (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61: 807–816.
Gray H (1918) Anatomy of the Human Body. Lea & Febiger: Philadelphia.
Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V et al (2006). The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry 60: 760–766.
Grupe DW, Nitschke JB (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14: 488–501.
Grupe DW, Oathes DJ, Nitschke JB (2013). Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex 23: 1874–1883.
Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG (2009). The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacology Biol Psychiatry 33: 1309–1320.
Harris AC, Gewirtz JC (2004). Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 171: 140–147.
Heimer L, Alheid GF (1991). Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol 295: 1–42.
Hitchcock J, Davis M (1986). Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci 100: 11–22.
Hitchcock JM, Davis M (1987). Fear-potentiated startle using an auditory conditioned stimulus: effect of lesions of the amygdala. Physiol Behav 39: 403–408.
Hitchcock JM, Davis M (1991). Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci 105: 826–842.
Hoffman KL, Gothard KM, Schmid MC, Logothetis NK (2007). Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol 17: 766–772.
Jamieson JP, Mendes WB, Nock MK (2013). Improving acute stress responses: the power of reappraisal. Curr Dir Psychol Sci 22: 51–56.
Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD (2013a). The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341: 1517–1521.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al (2013b). Distinct extended amygdala circuits for divergent motivational states. Nature 496: 224–228.
Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ (2005). Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry 58: 796–804.
Kash TL (2012). The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol 46: 303–308.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005a). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005b). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627.
Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS et al (2013). Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496: 219–223.
Kinnison J, Padmala S, Choi J, Pessoa L (2012). Network analysis reveals increased integration during emotional and motivational processing. J Neurosci 32: 8361–8372.
Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SEA, Oosting RS et al (2014). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biol Psychiatry 14: 1–8.
Koob GF (2009). Brain stress systems in the amygdala and addiction. Brain Res 1293: 61–75.
Koob GF, Moal MLe (1997). Drug abuse: hedonic homeostatic dysregulation. Science 278: 52–58.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Krüger O, Shiozawa T, Kreifelts B, Scheffler K, Ethofer T (2015). Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex 66: 60–68.
Lee Y, Davis M (1997). Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci 17: 6434–6446.
Lesur A, Gaspar P, Alvarez C, Berger B (1989). Chemoanatomic compartments in the human bed nucleus of the stria terminalis. Neuroscience 32: 181–194.
Logrip ML, Koob GF, Zorrilla EP (2011). Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs 25: 271–287.
Lovallo WR (2006). Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol 59: 195–202.
Lu L, Shepard JD, Hall FS, Shaham Y (2003). Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev 27: 457–491.
Lupien S, Mcewen B (1997). The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev 24: 1–27.
Mai J, Paxinos G, Voss T (2008) Atlas of the Human Brain. Elsevier: New York: New York.
McEwen BS (2012). Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA 109: 17180–17185.
McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004). Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24: 1551–1560.
McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, Pessoa L (2014). Network organization unfolds over time during periods of anxious anticipation. J Neurosci 34: 11261–11273.
Mechias ML, Etkin A, Kalisch R (2010). A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. Neuroimage 49: 1760–1768.
Miles L, Davis M, Walker D (2011). Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology 36: 1563–1574 Classic review of the pharmacological dissociation between ‘fear’ and ‘anxiety’ in rodents.
Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T (2010). Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci USA 107: 20582–20586 Demonstrated that the BNST tracks with spatial proximity of threat in humans, similar to the defensive behavior patterns observed in rodents.
Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M (2014). Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. J Neurosci 34: 10430–10437.
O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML et al (2012). Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology 37: 2267–2276.
Ohman A (1986). Face the beast and fear the face: animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology 23: 123–145.
Oler JA, Birn RM, Patriat R, Fox AS, Shelton SE, Burghy CA et al (2012). Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. Neuroimage 61: 1059–1066 First study to demonstrate functional connectivity between the BNST and central nucleus of the amygdala in both non-human primates and humans.
Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, Koob GF (2013). Corticotropin-releasing factor (CRF) and α 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. Int J Neuropsychopharmacol 16: 1867–1875.
Porrino LJ, Crane AM, Goldman-Rakic PS (1981). Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol 198: 121–136.
Price J (2003). Evolutionary aspects of anxiety disorders. Dialogues Clin Neurosci 5: 223–236.
Price JL, Amaral DG (1981). An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1: 1242–1259.
Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C et al (2008). Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 63: 234–240.
Ravinder S, Burghardt NS, Brodsky R, Bauer EP, Chattarji S (2013). A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Transl Psychiatry 3: e209.
Reynolds SM, Zahm DS (2005). Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci 25: 11757–11767.
Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC (2014). Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci 8: 1–19.
Robinson OJ, Overstreet C, Allen PS, Pine DS, Grillon C (2012). Acute tryptophan depletion increases translational indices of anxiety but not fear: serotonergic modulation of the bed nucleus of the stria terminalis? Neuropsychopharmacology 37: 1963–1971.
Schlund MW, Hudgins CD, Magee S, Dymond S (2013). Neuroimaging the temporal dynamics of human avoidance to sustained threat. Behav Brain Res 257: 148–155.
Schmitz A, Grillon C (2012). Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat Protoc 7: 527–532.
Shaham Y, Shalev U, Lu L, Wit H De, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20.
Shin LM, Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35: 169–191.
Silberman Y, Matthews RT, Winder DG (2013). A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci 33: 950–960.
Smart OL, Tiruvadi VR, Mayberg HS (2015). Multimodal approaches to define network oscillations in depression. Biol Psychiatry 77: 1061–1070.
Somerville LH, Whalen PJ, Kelley WM (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 68: 416–424 First study to demonstrate that BNST activity correlates with trait anxiety in humans.
Stamatakis AM, Sparta DR, Jennings JH, Mcelligott ZA, Decot H, Stuber GD (2014). Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology 76: 320–328.
Straube T, Mentzel H-J, Miltner WHR (2007). Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage 37: 1427–1436 One of the first human BNST studies; demonstrated that patients with a spider phobia have elevated BNST activity when exposed to spider stimuli.
Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128: 7–14.
Swerdlow NR, Geyer MA, Vale WW, Koob GF (1986). Corticotropin-releasing factor (CRF) potentiates acoustic startle reflex (ASR) in rats. Blockade by chlordiazepoxide. Psychopharmacology 88: 147–152.
Turner BH, Zimmer J (1984). The architecture and some of the interconnections of the rat’s amygdala and lateral periallocortex. J Comp Neurol 227: 540–557.
Villada C, Hidalgo V, Almela M, Salvador A (2014). Individual differences in the psychobiological response to psychosocial stress (trier social stress test): the relevance of trait anxiety and coping styles. Stress Health, (e-pub ahead of print; doi:10.1002/smi.2582).
Waddell J, Morris RW, Bouton ME (2006). Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci 120: 324–336.
Walker DL, Davis M (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci 17: 9375–9383 Seminal study demonstrating dissociation between amygdala and BNST in the production of ‘fear’ vs ‘anxiety’ behaviors.
Walker DL, Miles LA, Davis M (2009). Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacology Biol Psychiatry 33: 1291–1308.
Walker DL, Toufexis DJ, Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199–216.
Walter A, Mai JK, Lanta L, Görcs T (1991). Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. J Chem Neuroanat 4: 281–298.
Wang J, Fang Q, Liu Z, Lu L (2006). Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 185: 19–28.
Wenzel JM, Cotten SW, Dominguez HM, Lane JE, Shelton K, Su Z-I et al (2014). Noradrenergic -receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci 34: 3467–3474.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE et al (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586.
Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ et al (2012). GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci USA 109: E278–E287.
Yassa MA, Hazlett RL, Stark CEL, Hoehn-Saric R (2012). Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res 46: 1045–1052.
Zinbarg RE, Barlow DH (1996). Structure of anxiety and the anxiety disorders: a hierarchical model. J Abnorm Psychol 105: 181–193.
Acknowledgements
We thank Dr Danny Winder and Dr Meg Benningfield for their helpful suggestions. This work was supported by funding to the authors from the National Institutes of Mental Health (F31-MH102008 to SNA; F30-MH097344 to JAC; R21MH106998 to JUB), and the Vanderbilt Medical Scientist Training Program (NIGMS; T32-GM07347). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avery, S., Clauss, J. & Blackford, J. The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacol 41, 126–141 (2016). https://doi.org/10.1038/npp.2015.185
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.185
This article is cited by
-
Threat Responses in Schizophrenia: A Negative Valence Systems Framework
Current Psychiatry Reports (2024)
-
Subcortical serotonin 5HT2c receptor-containing neurons sex-specifically regulate binge-like alcohol consumption, social, and arousal behaviors in mice
Nature Communications (2023)
-
A sex-specific role for the bed nucleus of the stria terminalis in proactive defensive behavior
Neuropsychopharmacology (2023)
-
Defensive responses: behaviour, the brain and the body
Nature Reviews Neuroscience (2023)
-
BNST PKCδ neurons are activated by specific aversive conditions to promote anxiety-like behavior
Neuropsychopharmacology (2023)