Abstract
The current study examined whether adolescent rats are more vulnerable than adult rats to the lasting adverse effects of cannabinoid exposure on brain and behavior. Male Wistar rats were repeatedly exposed to Δ-9-tetrahydrocannabinol (Δ9-THC, 5 mg/kg i.p.) in a place-conditioning paradigm during either the adolescent (post-natal day 28+) or adult (post-natal day 60+) developmental stages. Adult rats avoided a Δ9-THC-paired environment after either four or eight pairings and this avoidance persisted for at least 16 days following the final Δ9-THC injection. In contrast, adolescent rats showed no significant place aversion. Adult Δ9-THC-treated rats produced more vocalizations than adolescent rats when handled during the intoxicated state, also suggesting greater drug-induced aversion. After a 10–15 day washout, both adult and adolescent Δ9-THC pretreated rats showed decreased social interaction, while only Δ9-THC pretreated adolescent rats showed significantly impaired object recognition memory. Seventeen days following their last Δ9-THC injection, rats were euthanased and hippocampal tissue processed using two-dimensional gel electrophoresis proteomics. There was no evidence of residual Δ9-THC being present in blood at this time. Proteomic analysis uncovered 27 proteins, many involved in regulating oxidative stress/mitochondrial functioning and cytoarchitecture, which were differentially expressed in adolescent Δ9-THC pretreated rats relative to adolescent controls. In adults, only 10 hippocampal proteins were differentially expressed in Δ9-THC compared to vehicle-pretreated controls. Overall these findings suggest that adolescent rats find repeated Δ9-THC exposure less aversive than adults, but that cannabinoid exposure causes greater lasting memory deficits and hippocampal alterations in adolescent than adult rats.
Similar content being viewed by others
INTRODUCTION
Cannabis sativa has long been used for recreational and medicinal purposes and remains one of the most widely used illicit drugs worldwide (Ashton, 2002; Iversen, 2003). Of some concern is the increasing number of young adolescent cannabis users (Substance Abuse and Mental Health Services Administration, 2005). Adolescence is increasingly viewed as an important developmental window, somewhat analogous to that of infancy (Powell, 2006). Ongoing neuroplastic modifications occurring in the adolescent central nervous system (CNS) include changes in dendritic spine density, synaptic rearrangements and development of myelination, and these changes are thought to support the emerging adult cognitive style (Chambers et al, 2003). This remodeling process may conceivably be disrupted by cannabinoids leading to lasting adverse effects on brain and behavior.
More than 20 years ago, residual learning impairments were reported in adolescent but not adult rats exposed to chronic cannabinoid treatment (Stiglick and Kalant, 1985). More recently, administration of the synthetic cannabinoid agonist WIN 55,212-2 during the adolescent but not the adult period was reported to cause long-lasting deficits in sensorimotor gating, object recognition memory, and performance on an instrumental task (Schneider and Koch, 2003; Schneider et al, 2005). Residual working memory and social interaction deficits were also found in adolescent but not adult female rats treated with the synthetic cannabinoid agonist CP 55,940 (O'Shea et al, 2004). In a human study, early- but not late-onset human cannabis users exhibited longer reaction times in a visual scanning task suggestive of a persistent attentional deficit (Ehrenreich et al, 1999). Overall, such results support the idea of a specific vulnerability of adolescents to the enduring adverse effects of cannabinoids on cognition.
The hippocampus may play a key role in mediating these long-term adverse effects of cannabinoids. There is a high density of CB1 cannabinoid receptors in the hippocampus (Herkenham et al, 1990) and injection of cannabinoids directly into this region impairs memory (Lichtman et al, 1995). A recent study indicates that cannabinoids desynchronize hippocampal neuronal assemblies and that this may be linked to cannabinoid-induced memory impairment (Robbe et al, 2006). Moreover, hippocampal morphological changes have been observed following chronic administration of cannabinoids (Lawston et al, 2000; Tagliaferro et al, 2006).
The apparent preponderance of cannabis use during adolescence might conceivably reflect a differential reinforcing effect of the drug when consumed at earlier developmental stages. This hypothesis has not to our knowledge been tested using animal models. Rats generally find cannabinoids aversive, with Δ-9-tetrahydrocannabinol (Δ9-THC) and synthetic cannabinoids producing conditioned place aversion (Parker and Gillies, 1995; McGregor et al, 1996; Sanudo-Pena et al, 1997; Mallet and Beninger, 1998; Cheer et al, 2000) and conditioned taste avoidance (Parker and Gillies, 1995; McGregor et al, 1996). Reports of dysphoria and panic by human cannabis users (Hall et al, 1994) suggest these aversive effects may sometimes have a human correlate.
Previous studies have utilized cDNA microarray to assess changes of gene expression profiles induced by cannabinoids in the CNS and suggest a variety of gene expression changes following chronic cannabinoid treatment (Kittler et al, 2000; Grigorenko et al, 2002; Parmentier-Batteur et al, 2002). However, to our knowledge, no previous study has analyzed brain protein expression profiles of chronic cannabinoid-exposed rats. The current study therefore aimed to assess whether Δ9-THC has differential aversive effects in adolescent and adult rats and whether there are greater lasting effects on memory and anxiety in rats given Δ9-THC during adolescence. Further, two-dimensional gel electrophoresis (2DE) proteomic analysis was conducted to assess whether Δ9-THC has residual effects on hippocampal protein expression and whether these effects are greater in adolescent rats than in adults.
MATERIALS AND METHODS
Subjects
The subjects were 48 experimentally naive male albino Wistar rats (Animal Resource Centre, Perth, Australia). Of these, 24 were 28 days old (adolescent) and 24 were 60 days old (adult) on delivery to our colony, consistent with the adolescent ontogenetic window of 28–55 days for rats (Spear, 2000). For each developmental cohort, 12 rats were chronically exposed to Δ9-THC with the other 12 serving as vehicle (VEH)-treated controls.
After arriving in the colony, rats were handled daily for 4 days before testing began. At the start of treatment, adolescent rats weighed 76.2±2.4 g and adults, 284±4.4 g. Rats had ad libitum access to food and water and were group-housed in a temperature- and humidity-controlled colony room maintained on a reverse 12-h light/12-h dark cycle. All testing took place during the dark cycle. All experiments were approved by the University of Sydney Animal Ethics Committee in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Drug Preparation and Administration
Δ9-THC in absolute ethanol (30 mg/ml, Sigma) was added to an equal volume of Tween 80 and diluted in sterile saline to give a final stock solution of ethanol/Tween-80/saline 1 : 1 : 10. All injections were administered i.p. at a volume of 2 ml/kg.
Rats in the drug-exposed groups received two initial 1 mg/kg doses of Δ9-THC across 2 days, while control rats received equivalent injections of VEH. Place preference or aversion may develop to a particular testing apparatus if cannabinoids are first experienced in this apparatus (Valjent and Maldonado, 2000). An initial priming dose in the home cage is thought to lessen this association (Viveros et al, 2005).
Behavioral Testing
All behavioral tests were performed in a dark room illuminated by a 60 W red lamp. Infrared-sensitive miniature video cameras with infrared illuminating light-emitting diodes (Jaycar Ltd, Australia, model QC3468) were suspended above the apparatus. An observer blind to group allocations manually scored trials, via remote video monitoring, using ODLog software (Macropod Software, 2001; www.macropodsoftware.com). All apparatus were wiped down with a 50% ethanol solution in between trials. A timeline of behavioral testing is presented in Table 1.
Place conditioning
Place conditioning was tested in an unbiased three-compartment apparatus. The four identical chambers (Med Associates, St Albans, VT) consisted of two side compartments (28 × 21 × 21 cm) separated by a center compartment (12 × 21 × 21 cm). Guillotine doors were placed at both entries to the center compartment to restrict access when necessary. The two side compartments were texturally and visually different and had different olfactory cues. One side had black and white striped walls, a grid mesh floor, and 1 ml of Cornwell's white vinegar in the waste pan beneath the floor. The other side had black walls, a metal rod floor, and 1 ml of Queen natural almond essence in the waste pan. Waste pans were washed with warm water and the olfactory cue reapplied in between each trial.
Testing began 2 days after the final priming injection and was conducted in five phases: baseline (day 1), first conditioning phase (days 2–9), test 1 (day 10), second conditioning phase (days 11–18), and test 2 (day 19). In the baseline phase, test 1 and test 2, rats were allowed to freely explore all compartments for 30 min and time spent in each compartment was scored.
The first conditioning phase consisted of eight 50 min sessions, one per day. For the two Δ9-THC groups, the drug was administered on even numbered days and VEH injections were given on odd days. For the two VEH groups, VEH injections were given every day. A 5 mg/kg Δ9-THC dose was chosen as it has previously been shown to induce memory impairment and is considered equivalent to heavy cannabis use in humans (Nakamura et al, 1991). Rats were placed in alternate sides of the chamber each day. At the conclusion of this phase, Δ9-THC had been paired with one context on four occasions and VEH with the other on four occasions. The drug-associated context (striped side or black side) was counterbalanced across groups. The second conditioning phase was identical to the first in all ways except that Δ9-THC was administered on odd days and VEH on even days.
During the first conditioning phase, it was noted that adult Δ9-THC-treated rats often emitted audible vocalizations when handled by the experimenter. These vocalizations were systematically recorded for all groups during the last five drug/context pairings as an additional index of cannabinoid-induced aversion (Henriksson and Jarbe, 1971; Giuliani et al, 2000).
Following a 16-day drug-free period, rats were once again given free access to the test chamber for 30 min to test the persistence of any conditioned place preference or aversion. Time spent in each compartment was again scored.
Acute Δ9-THC effects: the emergence test
Three days following the completion of place preference test 2, rats were assessed for the acute effects of Δ9-THC on anxiety-like behavior using a behavioral battery that consisted of the emergence, elevated plus maze (EPM), and social interaction tests (Morley et al, 2001). Rats were administered 5 mg/kg Δ9-THC or VEH and 20 min later were placed in either the emergence test or EPM for 5 min. They were then placed in the other apparatus (EPM or emergence) for 5 min. The order in which these two tests were conducted was counterbalanced across groups.
The emergence test consisted of a 120 × 120 × 45 cm white melamine arena with a 25 × 40 × 18 cm black melamine hide box and was conducted as described previously (Morley et al, 2001). The rat was placed in the hide box at the beginning of a 5 min test period. Scored behaviors included latency to emerge, risk assessment time (defined as front paws and head protruding from the hide box), number of risk assessment behaviors, time spent in the open field, and number of open-field entries.
Acute Δ9-THC effects: the elevated plus maze
The apparatus consisted of two open and two closed 10 × 50 cm arms arranged in a cross-elevated position, 53 cm off the ground. Scored behaviors included percentage time spent in the open arm as a function of total arm time, time spent rearing, and total number of entries into the closed arm and center square.
Acute Δ9-THC effects: social interaction test
The social interaction test was conducted 5 min after the emergence and EPM tests and was conducted as described previously (File and Seth, 2003) in a 50 × 50 × 40 cm black melamine box. Rats were placed in the apparatus for 10 min with treatment- and age-matched conspecifics of approximately the same body weight but from a different home cage. Scored behaviors included general investigation, anogenital sniffing, adjacent lying, and rearing.
Residual Δ9-THC effects: novel object recognition
Following a 10-day drug-free period rats were tested in a novel object recognition (NOR) task (Ennaceur and Delacour, 1988). The experimental chamber consisted of a black circular plastic arena of 75 cm diameter with a 40 cm high wall. The day before testing, rats were habituated to the test chamber for 10 min. Formal testing was conducted over the next 2 days. Rats were first placed in the chamber with two identical objects for 3 min (T1). Objects used included flowerpots, door handles, drink bottles, and flasks. The assignment of objects was counterbalanced and equally distributed across groups, as was the location of the novel object during the test phase (to the left or right of the arena). After 1 h, rats were again placed in the chamber with two objects for 3 min. On this second trial (T2), one object was an identical copy of the object presented previously while the other was novel. Object exploration was scored when the rat's snout was directed within 2 cm of the object. Climbing over or sitting on the object was not recorded as exploration.
Residual Δ9-THC effects: social interaction
After 15-day drug-free period, rats were once again assessed in the social interaction paradigm. Testing was conducted as described above however the rats were paired with a different age- and treatment-matched conspecific.
Euthanasia and Determination of Blood Δ9-THC Levels
Rats were euthanased by decapitation, using a guillotine, 17 days after the last drug treatment. Brains were removed and the hippocampi rapidly dissected out and kept at −80°C until use.
Trunk blood samples were collected for four rats in each treatment group immediately after decapitation and for an additional group of age-matched adult (n=8) and adolescent (n=6) male Wistar rats given a 1 mg/kg challenge dose of Δ9-THC 30 min before being euthanased.
Determination of whole blood concentrations of Δ9-THC and its major metabolite Δ9-THC-COOH was conducted by gas chromatography–mass spectrometry (GC–MS) using selected ion monitoring with deuterium-labeled (d3) Δ9-THC and Δ9-THC-COOH as the internal standards. Sample preparation involved extraction of an acidic sample using hexane:ethyl acetate (9 : 1) and evaporation of the extract to dryness. The residue was derivatized with bis(trimethylsilyl)trifluoroacetamide 99+1% trimethylchlorosilane:hexane (1 : 5) and injected onto the GC–MS. Spiked blood samples were used to construct a calibration curve (Δ9-THC, 0.005–0.050 mg/L; Δ9-THC-COOH, 0.010–0.100 mg/L) for calculation of the unknown Δ9-THC and Δ9-THC-COOH levels.
2DE Proteomics
The hippocampi of 24 rats (n=6 per group) were used for proteomic analysis. Protein extraction and gel analysis of hippocampal samples were conducted as described previously (Iwazaki et al, 2006). Briefly, protein was extracted from frozen brain tissue (approximately 50 mg) and then resuspended in 1 ml of buffer consisting of 7 M urea, 2 M thiourea, and 1% C7bZO. Extracted protein was assayed using the Bradford method (Bradford, 1976) to determine protein concentration. 2DE proteomics was then performed by isoelectric focusing of immobilized pH gradient strips and separation on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels using the ElectrophoretIQ3 system (Proteome Systems). Gels were fixed in a solution containing 25% (v/v) methanol and 10% (v/v) acetic acid for 1 h and then stained using colloidal Coomassie blue.
The resulting 48 gels (duplicate runs for each sample) were digitized at 400 dots per inch using a transmissive flatbed scanner (UMAX) and analyzed using Phoretix 2D Expression software (nonlinear). Differences in spot intensity of averaged gels for each treatment group were then analyzed using t-tests. Protein spots of interest were excised from gels and analyzed using an Applied Biosystems QSTAR MALDI-TOF mass spectrometer. The obtained MALDI spectra were searched against the Swiss-prot protein database using the MASCOT search engine (http://www.matrixscience.com). Modifications of oxidation and propionamide were considered and up to one missed cleavage was permitted. Positive protein identification was defined when the Mowse score was significant (>50, rattus database) and isoelectric point (pI) and molecular weight (MW) values matched those estimated from the gels. Identified proteins were further searched using the PathwayArchitect database (Stratagene) for identification of protein–protein interactions and pathway analysis.
Statistical analysis
Percent of time spent in the drug-paired side in the place-conditioning apparatus, percentage of time spent exploring the novel object, and all anxiety measures were compared between groups using planned contrasts (Winer et al, 1991). Three main contrasts were of interest: (1) the adolescent Δ9-THC group vs adolescent VEH group, (2) the adult Δ9-THC group vs adult VEH group, and (3) all Δ9-THC-treated rats (adolescent plus adult) vs all VEH-treated rats.
The presence of handling-induced vocalizations in adult and adolescent rats during drug treatment in the place-conditioning paradigm was compared using a χ2-test.
All analyses were conducted using SPSS 12 for Windows with the level of significance set at 0.05.
RESULTS
During the course of experimentation, three rats were removed due to illness. These included one adolescent VEH-treated rat and one adolescent Δ9-THC-treated rat removed during the second conditioning phase of place conditioning, and one adolescent VEH-treated rat removed during the drug-free period.
Place Conditioning
For the baseline test, data from four rats were lost due to a computer problem. No overall treatment effect (F1,40=1.46, p>0.05) nor any adolescent (F1,40=0.36, p>0.05) or adult (F1,40=1.23, p>0.05) group effects were seen for baseline place preference.
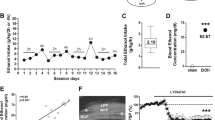
After four drug pairings there was no overall significant effect of Δ9-THC treatment (F1,44=3.57, p=0.07). No significant treatment effect was observed for adolescent rats (F1,44=0.02, p>0.05) while adult Δ9-THC-treated rats spent significantly less time in the drug-paired compartment than adult controls (F1,44=6.39, p<0.05) (Figure 1).
After eight drug pairings Δ9-THC-treated rats considered together spent less time in the drug-paired side relative to controls (F1,42=15.99, p<0.001). Adult Δ9-THC-treated rats spent significantly less time in the drug-paired side than adult controls (F1,42=15.66, p<0.001) whereas adolescent Δ9-THC rats did not differ significantly from controls (F1,42=3.05, p>0.05) (Figure 1).
Over the last five conditioning sessions involving Δ9-THC treatment, a greater number of adult rats emitted audible vocalizations than adolescent rats when handled in the intoxicated state (χ12=14.44, p<0.001) (Table 2). No vocalizations were observed on VEH treatment days or in any of the VEH-group rats.
When place aversion was retested after the 16-day drug-free period, there remained a significant overall drug treatment effect (F1,39=4.83, p<0.05). Adult Δ9-THC-treated rats maintained an aversion to the drug-paired side relative to adult VEH-treated rats (F1,39=5.42, p<0.05) while adolescent Δ9-THC-treated rats did not differ significantly from adolescent VEH-treated rats (F1,39=0.64, p>0.05) (Figure 1).
Acute Δ9-THC Effects: The Emergence Test
Δ9-THC-treated rats took longer to emerge from the hide box (F1,42=7.81, p<0.01), displayed more risk assessment behaviors (F1,42=24.26, p<0.001), spent less time in the open field (F1,42=9.98, p<0.01), and entered the open field on fewer occasions (F1,42=7.80, p<0.01) than VEH-treated rats.
Δ9-THC-treated adolescent rats had a significantly greater latency to emerge (F1,42=6.39, p<0.05), more risk assessment behaviors (F1,42=8.60, p<0.01), and spent less time in the open field (F1,42=6.82, p<0.05) than adolescent VEH-treated rats. In adult rats, Δ9-THC-treated rats performed significantly more risk assessment behaviors (F1,42=16.48, p<0.001) and showed significantly less entries into the open field (F1,42=6.17, p<0.05) (Table 3).
Acute Δ9-THC Effects: The Elevated Plus Maze
Δ9-THC-treated rats spent a lesser percentage time in the open arm (F1,42=8.21, p<0.01) than controls and spent less time rearing (F1,42=36.02, p<0.001). Adolescent Δ9-THC-treated rats had significantly lower percentage time on the open arm (F1,42=7.76, p<0.01) than adolescent controls and spent significantly less time rearing (F1,42=6.86, p<0.05). In adult rats, effects of Δ9-THC were only significant for rearing time (F1,42=35.32, p<0.001). There were no significant drug effects on total number of arm entries (Table 4).
Acute Δ9-THC Effects: Social Interaction
Overall, Δ9-THC-treated rats spent less time engaged in social interaction than VEH controls (F1,19=6.17, p<0.05). No significant differences in total social interaction were found between Δ9-THC- and VEH-treated adolescent (F1,19=3.93, p=0.06) nor adult rats (F1,19=2.31, p>0.05). A significant overall treatment effect was found for time spent rearing (F1,19=22.58, p<0.001) with decreased rearing in both adolescent (F1,19=5.90, p<0.05) and adult (F1,19=18.82, p<0.001) Δ9-THC-treated rats (Figure 2).
Residual Δ9-THC Effects: Novel Object Recognition
Time spent exploring objects during the sample phase was similar in adolescent VEH (40.52±3.24), adolescent Δ9-THC (44.65±2.47), adult VEH (38.10±2.68), and adult Δ9-THC (36.86±2.80) groups.
A significant main effect of treatment was observed on the percentage of time spent investigating the novel object during T2 (F1,41=12.75, p<0.01). Adolescent Δ9-THC pretreated rats showed a significant decrease in percentage of time spent investigating a novel object compared to adolescent controls (F1,41=12.37, p<0.01). In adult rats, there was no significant difference between Δ9-THC pretreated rats and their controls (F1,41=2.15, p>0.05) (Figure 3).
Residual Δ9-THC Effects: Social Interaction
After the drug-free period, Δ9-THC pretreated rats spent less time in social interaction than controls (F1,19=19.80, p<0.001). This decreased social interaction was seen in both adolescent (F1,19=14.38, p<0.01) and adult Δ9-THC-pretreated rats (F1,19=6.11, p<0.05) (Figure 4). No significant differences in rearing were observed (Table 4).
Residual Blood Δ9-THC Levels
GC–MS analysis of trunk blood collected 17 days after the last drug administration showed no detectable levels of Δ9-THC and only minimal amounts of the Δ9-THC metabolite Δ9-THC-COOH. By comparison, rats given an acute 1 mg/kg dose of Δ9-THC 30 min before blood collection showed detectable levels of both Δ9-THC and Δ9-THC-COOH (Table 5).
Hippocampal Protein Expression Profiles
Significant alterations in protein expression in the hippocampus of adolescent and adult Δ9-THC-pretreated rats were established by comparison with VEH-treated averaged gels for each age group. The averaged gels for adolescent VEH, adolescent Δ9-THC, adult VEH, and adult Δ9-THC groups displayed 431, 457, 453, and 520 matched spots respectively.
Figure 5 shows a typical 2DE gel pattern of protein extracts for the hippocampus of adolescent rats. In these adolescent samples, 27 spots were differentially regulated in Δ9-THC-pretreated rats (p<0.05). A detailed image of spot 558, identified as phosphoglycerate mutase 1 (PGAM1), shows it is downregulated in Δ9-THC pretreated adolescent animals as determined from the normalized volumes; whereas spot 604, identified as Ras-related protein Rab-1A, is upregulated in Δ9-THC-pretreated adolescent animals. Of the 27 differentially regulated spots, 24 (89%) showed decreased expression in Δ9-THC pretreated adolescent rats compared to controls. Twelve spots showed a 1.5- to 2-fold decrease, and 12 spots showed a less than 1.5-fold decrease. Three spots showed an increased expression in Δ9-THC-pretreated rats with all three in the range of 1.5- to 2-fold.
(a) A typical 2DE gel image of expressed proteins in the adolescent rat hippocampus. Intact circles indicate spots of decreased expression and broken lines indicate spots of increased expression in adolescent THC-treated rats. MW, molecular weight. The side panel shows a magnified image of spot 558 identified as PGAM1 and spot 604 identified as Ras-related protein Rab-1A. (b) The normalized volume of spot 558 was significantly decreased whereas the normalized volume of spot 604 was increased in the Δ9-THC pretreated group (**p<0.01).
In adult hippocampal samples, 10 spots were differentially regulated in Δ9-THC pretreated rats (p<0.05). Of these, nine (90%) showed a decrease in expression in Δ9-THC samples compared to controls. Two spots showed decreased expression greater than 2-fold, four spots a 1.5- to 2-fold decrease, and three spots less than 1.5-fold decrease. One spot showed an increase in expression that was 1.5- to 2-fold.
Identification of Altered Proteins
All differentially regulated spots were analyzed by MALDI-TOF and 19 were identified from the adolescent samples and 8 from the adult samples. A summary of the identified proteins for adolescent and adult samples is shown in Tables 6 and 7, respectively. Spots 550, 551, 554, and 558, identified as PGAM1, and spots 439 and 447, identified as NAD-dependent deacetylase sirtuin-2, were identified as the same protein respectively. These may represent different protein isoforms resulting from post-translational modifications. Spots 239 and 447 were identified as mixtures of tubulin α-2 chain and tubulin β-3 chain, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and NAD-dependent deacetylase sirtuin-2, respectively.
DISCUSSION
The main findings from the current study are that (1) adolescent rats appear to find Δ9-THC less aversive than adult rats, (2) Δ9-THC causes a residual impairment in object recognition memory in adolescent but not adult rats, (3) Δ9-THC may have similar long-lasting adverse effects on social interaction in both adolescent and adult rats, and (4) there is a more profound lasting impact of Δ9-THC on protein expression profiles in the hippocampus of adolescent compared to adult rats.
Acute Aversive Effects of THC
This work confirms previous findings that cannabinoid agonists are aversive to rats, as shown by the formation of a conditioned place aversion (McGregor et al, 1996; Sanudo-Pena et al, 1997; Mallet and Beninger, 1998; Cheer et al, 2000). Δ9-THC-treated adult rats avoided the drug-paired side after both four and eight contextual pairings, while adolescent rats only showed a trend toward place aversion, and only after eight pairings. Moreover, adult, but not adolescent, rats still avoided a Δ9-THC-paired context 16 days following the last drug exposure. While these effects might also be explained by a greater disruptive effect of Δ9-THC on contextual conditioning in adolescent than adult rats, a greater aversion to Δ9-THC in adult rats is also indicated by the larger number of adult Δ9-THC-treated rats emitting audible vocalizations during handling. Tactile stimulus-elicited vocalization is well documented in cannabinoid-treated rats and is considered an index of a negative drug-induced emotional state (Henriksson and Jarbe, 1971; Giuliani et al, 2000).
The reduced aversive effects of cannabinoids in adolescent rats parallel analogous developmental effects observed with alcohol (Philpot et al, 2003) and nicotine (Wilmouth and Spear, 2004). This suggests that certain characteristics of the still-developing adolescent brain may make adolescent rats less sensitive to the use-limiting aversive properties of various drugs of abuse and hence more vulnerable to continued use and addiction.
Acute Anxiogenic Effects of THC
Acute Δ9-THC administration (5 mg/kg), after repeated Δ9-THC exposure in the place preference paradigm, had anxiogenic effects in the emergence test (decrease open-field time, increased risk assessment, increased emergence latency), EPM test (decreased percentage open-arm time), and social interaction test (decreased interaction with a novel conspecific). No substantial differential anxiogenic effects of Δ9-THC could be discerned in adult and adolescent rats although this conclusion is not definitive given the single dose of Δ9-THC that was tested.
The observed anxiogenic effects of cannabinoids agree with previous results in the emergence and EPM tests (Rodriguez de Fonseca et al, 1996; Arevalo et al, 2001; Caberlotto et al, 2004; Marco et al, 2004) and the social interaction test (Genn et al, 2004). Adolescent behavior in rodents is characterized by greater social interaction and exploratory behavior relative to adults (Spear, 2000) and such effects were evident in control rats in the social interaction and EPM tasks in the present study. Hence, it is most likely that the higher open-arm time at baseline in the adolescent rats allowed a greater margin for reduction by acute Δ9-THC treatment.
Cannabinoid agonists have a well-documented effect of suppressing locomotor activity at higher doses (Howlett et al, 2002) which was reflected in some of the present results, including decreased rearing in the EPM and social interaction tests and reduced open-field entries in the emergence test. However, there were no obvious signs of catalepsy in the rats tested on these models, so it is unlikely that observed anxiogenic effects were confounded by gross motor impairment.
Finally, it is worth noting that the anxiety tests were conducted at the very end of the adolescent period when the adolescent and adult rats were 55 and 87 days old, respectively. Further testing at an earlier developmental period with a full dose–response function for Δ9-THC may uncover differential anxiogenic effects of Δ9-THC in adolescent vs adult rats.
Residual Behavioral Effects
One of the most notable findings in the present study was the marked residual effects of Δ9-THC on social interaction and NOR approximately 2 weeks following the cessation of Δ9-THC treatment. These effects are unlikely to reflect persistence of pharmacologically relevant blood concentrations of Δ9-THC as GC–MS analysis showed that Δ9-THC had reached undetectable levels around this time and Δ9-THC-COOH, although detectable, was far below the level measured following a very modest (1 mg/kg) acute dose of Δ9-THC.
The residual decline in social interaction in Δ9-THC-pretreated rats agrees with a recent report of long-lasting reductions in social interaction arising from prior cannabinoid (CP 55,940) exposure in male adolescent and adult rats (O'Shea et al, 2006). A different pattern of results may have been obtained in female rats however, as an earlier study by the same authors reported an adolescent-specific social interaction deficit in cannabinoid pretreated female rats (O'Shea et al, 2004).
Strikingly, adolescent Δ9-THC-pretreated rats spent a decreased percentage of time relative to controls investigating a novel object, suggesting working memory dysfunction (Ennaceur and Delacour, 1988). Overall exploration times were not affected by prior Δ9-THC treatment, suggesting this deficit cannot be attributed to any nonspecific impairment or lack of exploration. This adolescent-specific deficit in object recognition memory is consistent with reports that cannabinoid administration in immature but not mature rats results in lasting impairments in learning (Stiglick and Kalant, 1985) and also more direct adolescent to adult comparisons showing adolescent-specific impaired object recognition memory after cannabinoid treatment (Schneider and Koch, 2003; O'Shea et al, 2004). This is also consistent with human studies showing persistent attentional deficits in early- but not late-onset cannabis users (Ehrenreich et al, 1999).
Changes in Protein Expression Profiles: Overview and Relationship to Behavior
2DE proteomics conducted on hippocampal samples revealed several proteins showing long-lasting alterations in response to Δ9-THC administration. The greater number of differentially expressed protein spots in adolescent Δ9-THC pretreated rats compared to adult Δ9-THC-pretreated rats suggests a greater vulnerability to lasting effects of Δ9-THC in the former group. Differentially expressed proteins in the hippocampus of Δ9-THC preexposed adolescents have a variety of functions broadly related to oxidative stress, mitochondrial and metabolic function, and regulation of the cytoskeleton and signaling.
In our laboratory, protein expression profiles have previously been measured in the rat striatum after chronic exposure to the atypical antipsychotic drug risperidone (O'Brien et al, 2006), acute (Iwazaki et al, 2006) and chronic methamphetamine exposure (Iwazaki et al, 2007), and in the hippocampus of human alcoholics (unpublished observations). When the present findings were compared to these data, only five proteins overlapped: PGAM1, triosephosphate isomerase (TPI), annexin A5, protein DJ-1, and β-tubulin. This indicates the protein modifications described here may be largely cannabinoid specific.
These protein modifications are more likely to be related to the lasting residual cognitive (object recognition) and behavioral (social interaction) changes seen in cannabinoid-pretreated rats rather than the acute cannabinoid effects (place aversion, acute anxiety) also reported here. While the hippocampus does play a role in the acquisition of contextual conditioning to various drugs (Meyers et al, 2006; Sharifzadeh et al, 2006), forming a link between lesser place aversion to THC in adolescent rats and hippocampal proteomic changes is difficult given that proteomic analysis occurred 3 weeks after place conditioning and at a time when the adolescent rats had reached adulthood. Analysis of the proteomic effects of acute cannabinoid treatment in adolescent and adult rats would clearly be an interesting follow-up study and might usefully examine nonhippocampal sites implicated in cannabinoid-reward, such as mesolimbic regions (Zangen et al, 2006).
In contrast to the place conditioning results, the greater residual impairment in object recognition memory in adolescent-pretreated rats can be readily linked to hippocampal protein changes. It is well demonstrated that the hippocampus proper, as well as adjacent cortical regions, plays a key role in object recognition memory. Hippocampal lesions impair object recognition memory as does temporary inactivation of the dorsal hippocampus using muscimol or post-training blockade of hippocampal protein synthesis using anisomycin (Broadbent et al, 2004; de Lima et al, 2006; Rossato et al, 2007). Thus, a link between greater residual memory impairment in adolescent-pretreated rats and greater hippocampal proteomic changes is plausible. Future studies employing a wider battery of cognitive tests may extend the characterization of these residual cognitive deficits into other hippocampus-relevant domains. An association might also be made between greater hippocampal proteomic changes in adolescent rats and their lack of any long-term retention of place aversion to THC.
Possible links between observed cannabinoid-induced social interaction deficits and hippocampal proteomic changes can also be considered. The hippocampus is generally considered to have a role in stress and anxiety responses (Gray and McNaughton, 2000) and both hippocampal lesions and infusion of specific drugs directly into the hippocampus can profoundly affect social interaction in rats (Becker et al, 1999; File et al, 2000; Deacon et al, 2002). However, given that equivalent social interaction deficits were seen in adult and adolescent THC-pretreated rats in the face of different hippocampal proteomic outcomes, it is likely that the link between social deficits and hippocampal changes is not so direct as is the case for object recognition memory deficits.
Protein Expression Profiles in Adolescent Brains
Differentially expressed proteins in Δ9-THC-pretreated adolescents that can be related to degenerative and oxidative changes included the stress-70 protein (GRP75), 60 kDa heat shock protein (HSP60), heat shock cognate 71 kDa protein (HSC71), and ubiquitin-conjugating enzyme E2. Heat shock proteins (GRP75, HSP60, and HSC71), which are found in the mitochondrion, are members of the chaperonin family that recognize nonnative conformations of other proteins and are essential in their folding and assembly (Takashima et al, 2003). They may also protect denatured proteins from aggregation and then promote refolding (Sargent et al, 1989). Microarray studies with cannabinoids have reported altered expression of related mRNAs, including heat shock 27 kDa protein 3 (Parmentier-Batteur et al, 2002) and 70 kDa heat shock protein (Kittler et al, 2000; Grigorenko et al, 2002). A disruption in the expression of these proteins could promote degenerative changes. Furthermore, glutathione-synthesizing enzymes, such as glutathione transferase ω-1, promote free radical scavenging (Chen et al, 2001). Reductions in these enzymes may make neurons more vulnerable to oxidative stress, which is associated with cognitive decline in aged animals (Poon et al, 2006) and may relate to memory deficits associated with cannabinoid exposure (Chan et al, 1998).
Ubiquitin-conjugating enzyme E2 is also involved in protein modification along with cellular proliferation and regulation of DNA repair. Previous studies have noted altered regulation of ubiquitin-conjugating enzyme 24 h after Δ9-THC administration (Kittler et al, 2000; Grigorenko et al, 2002) and the current study extends this to 17 days posttreatment. Expression of this protein may indicate disruption of cell proliferation and repair resulting from drug exposure during the adolescent period. Interestingly, genes for ubiquitin-conjugating enzymes are also decreased in human schizophrenic hippocampal samples (Altar et al, 2005). Cannabis use has been associated with a deteriorated course of schizophrenic illness and may indeed play a causal role in some cases of schizophrenia (Arseneault et al, 2002).
Differentially expressed proteins in adolescent Δ9-THC-exposed rats included cytoskeletal and other structural proteins, including transgelin-3 (NP25), α- and β-tubulin and myelin basic protein (MBP). This may be linked to structural changes or remodeling occurring after Δ9-THC exposure in adolescents and is consistent with observations of cytoarchitectural changes occurring with cannabinoid treatment (Tagliaferro et al, 2006) and other reports of altered expression of the structural-related proteins tubulin and actin (Tahir et al, 1992; Wilson et al, 1996). MBP is a major constituent of the myelin sheath that allows fast saltatory conduction of nerve pulses (Vanrobaeys et al, 2005). Previous studies employing microarray techniques have demonstrated downregulated MBP expression immediately after chronic Δ9-THC administration (Kittler et al, 2000; Grigorenko et al, 2002). Again, the current study is of interest as it shows such effects more than 2 weeks after Δ9-THC exposure, and only in rats exposed to Δ9-THC during the adolescent period.
The neuron-specific protein NP25 also showed altered expression in adolescent Δ9-THC-pretreated rats, again consistent with previous studies (Parmentier-Batteur et al, 2002). NP25 has a high homology with human neuronal protein (hNP22) which is implicated in morphological changes occurring after chronic alcohol exposure (Depaz et al, 2003) and in schizophrenia (Ito et al, 2005). Extending from its homology with hNP22, NP25 may colocalize with actin and tubulin. Altered expression of both NP25 and tubulin may reflect a modification in the microtubule cytoskeleton. Moreover, the suggested role of NP25 in synapse formation (Depaz and Wilce, 2006) indicates that disrupted NP25 signaling could account for the altered neuronal cytoskeleton and synaptic contacts seen after chronic WIN 55,212-2 treatment (Lawston et al, 2000; Tagliaferro et al, 2006). Decreases in Ras-related protein Rab-1A may also be linked to dysregulation in synaptic function. Members of the Ras family are implicated in the intracellular membrane trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptors underpinning long-lasting synaptic plasticity (Gerges et al, 2005). Ras protein alterations are thought to cause memory consolidation deficits (Brambilla et al, 1997). Together with disturbed neural cytoarchitecture and connectivity resulting from NP25 alterations, and increased oxidative damage, this could further account for adolescent-specific memory deficits.
Protein Expression Profiles in Adult Brains
Nine differentially expressed proteins were identified in adult rats following repeated Δ9-THC treatment. Only three of these (14-3-3 protein, glutathione transferase, and NAD-dependent deacetylase sirtuin-2) overlapped with those differentially expressed in adolescent Δ9-THC pretreated rats, suggesting divergent effects of the drug on adolescent and adult hippocampal proteomes. Differentially expressed proteins in adults were mostly enzymes involved in metabolic processes. This may indicate that Δ9-THC effects in adults are more transient-state-dependent alterations rather than persistent structural changes and that this may provide an underlying mechanism for the greater lasting adverse effects of the drug in adolescent rats.
Altered expression of aconitate hydratase (aconitase) and succinate dehydrogenase suggests a modification in the Krebs cycle. This pathway involves the oxidation of fuel molecules and is linked to glycolysis which provides the pyruvate for entry into Krebs cycle (Zatta et al, 2000). Two enzymes involved in glycolysis, GAPDH and TPI, also showed differential expression. Consistent with the current study, GAPDH has previously been shown to be downregulated after chronic cannabinoid exposure (Grigorenko et al, 2002). These alterations in both the Krebs cycle and glycolytic pathway indicate some impairment in oxidative and energy metabolism, perhaps due to compromised mitochondrial metabolism.
Serine/threonine protein phosphatase 2B, also referred to as calcineurin, was also significantly altered after adult Δ9-THC-exposure. Calcineurin has an important role in the control of intracellular Ca2+ signaling and may be involved in the desensitization of postsynaptic NMDA-receptor-coupled calcium channels (Guerini, 1997; Price and Mumby, 1999). Its expression is downregulated in Alzheimer's disease (Ladner et al, 1996).
Decreased glutathione S-transferase (GST) levels could impair detoxification processes targeted against age-associated oxidative stresses (Tchaikovskaya et al, 2005). GST enzymatic activity helps protect against toxic electrophiles. A similar glutathione transferase downregulation in Δ9-THC pretreated adolescent brains indicates a similar adverse effect of chronic THC on mechanisms that protect against oxidative stress. Hence, the apparent vulnerability associated with early-onset cannabis use could be due to earlier disruption of this protection mechanism resulting in more opportunity for oxidative stress.
CONCLUSIONS
The present study suggests that adolescent rats find Δ9-THC less aversive than adults, but that cannabinoid exposure has greater potential to cause lasting memory deficits and alterations in hippocampal structure/function in adolescent compared to adult rats. Proteins identified as differentially expressed in the adolescent brain give insight into possible mechanisms accounting for the adolescent-specific memory deficits. These age-specific differences emphasize the continual maturation of the adolescent brain and its vulnerability to long-term changes as a result of drug exposure. Future studies assessing long-term effects of various drugs of abuse would benefit from assessing age-specific effects. This is of particular importance due to the increasing prevalence of drug use in human populations in early adolescent stages.
References
Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y et al (2005). Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry 58: 85–96.
Arevalo C, de Miguel R, Hernandez-Tristan R (2001). Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav 70: 123–131.
Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE (2002). Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Br Med J 325: 1212–1213.
Ashton H (2002). Cannabis or health? Curr Opin Psychiatry 15: 247–253.
Becker A, Grecksch G, Bernstein HG, Hollt V, Bogerts B (1999). Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology 144: 333–338.
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE et al (1997). A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature 390: 281–286.
Broadbent NJ, Squire LR, Clark RE (2004). Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA 101: 14515–14520.
Caberlotto L, Rimondini R, Hansson A, Eriksson S, Heilig M (2004). Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: evidence for an allostatic shift? Neuropsychopharmacology 29: 15–22.
Chambers RA, Taylor JR, Potenza MN (2003). Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 160: 1041–1052.
Chan GC-K, Hinds TR, Impey S, Storm DR (1998). Hippocampal neurotoxicity of Delta-9-tetrahydrocannabinol. J Neurosci 18: 5322–5332.
Cheer JF, Kendall DA, Marsden CA (2000). Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology 151: 25–30.
Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA (2001). Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem 77: 1601–1610.
de Lima MN, Luft T, Roesler R, Schroder N (2006). Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett 405: 142–146.
Deacon RM, Bannerman DM, Rawlins JN (2002). Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav Neurosci 116: 494–497.
Depaz I, Ito M, Matsumoto I, Niwa S-i, Kroon P, Wilce PA (2003). Expression of hNP22 is altered in the frontal cortex and hippocampus of the alcoholic human brain. Alcohol Clin Exp Res 27: 1481–1488.
Depaz IM, Wilce PA (2006). The novel cytoskeleton-associated protein neuronal protein 22: elevated expression in the developing rat brain. Brain Res 1081: 59–64.
Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L et al (1999). Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology 142: 295–301.
Ennaceur A, Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31: 47–59.
File SE, Kenny PJ, Cheeta S (2000). The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66: 65–72.
File SE, Seth P (2003). A review of 25 years of the social interaction test. Eur J Pharmacol 463: 35–53.
Genn RF, Tucci S, Marco EM, Viveros MP, File SE (2004). Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacol Biochem Behav 77: 567–573.
Gerges NZ, Brown TC, Correia SS, Esteban JA (2005). Analysis of Rab protein function in neurotransmitter receptor trafficking at hippocampal synapses. Methods Enzymol 403: 153–166.
Giuliani D, Ferrari F, Ottani A (2000). The cannabinoid agonist HU 210 modifies rat behavioural responses to novelty and stress. Pharmacol Res 41: 47–53.
Gray JA, McNaughton N (2000). The Neuropsychology of Anxiety. Oxford University Press: Oxford.
Grigorenko E, Kittler J, Clayton C, Wallace D, Zhuang S, Bridges D et al (2002). Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chem Phys Lipids 121: 257–266.
Guerini D (1997). Calcineurin: not just a simple protein phosphatase. Biochem Biophys Res Comm 235: 271–275.
Hall W, Solowij N, Lemon J (1994). The Health and Psychological Consequences of Cannabis Use. Australian Government Publishing Services: Canberra.
Henriksson BG, Jarbe T (1971). Cannabis-induced vocalization in the rat. J Pharm Pharmacol 23: 457–458.
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR et al (1990). Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87: 1932–1936.
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA et al (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161–202.
Ito M, Depaz I, Wilce P, Suzuki T, Niwa S-i, Matsumoto I (2005). Expression of human neuronal protein 22, a novel cytoskeleton-associated protein, was decreased in the anterior cingulate cortex of schizophrenia. Neurosci Lett 378: 125–130.
Iversen L (2003). Cannabis and the brain. Brain 126: 1252–1270.
Iwazaki T, McGregor IS, Matsumoto I (2006). Protein expression profile in the striatum of acute methamphetamine-treated rats. Brain Res 1097: 19–25.
Iwazaki T, McGregor IS, Matsumoto I (2007). Protein expression profile in the striatum of rats with methamphetamine-induced behavioral sensitization. Proteomics 7: 1131–1139.
Kittler JT, Grigorenko EV, Clayton C, Zhuang SY, Bundey SC, Trower MM et al (2000). Large-scale analysis of gene expression changes during acute and chronic exposure to [Delta]9-THC in rats. Physiol Genomics 3: 175–185.
Ladner CJ, Czech J, Maurice J, Lorens SA, Lee JM (1996). Reduction of calcineurin enzymatic activity in Alzheimer's disease: correlation with neuropathologic changes. J Neuropathol Exp Neurol 55: 924–931.
Lawston J, Borella A, Robinson JK, Whitaker-Azmitia PM (2000). Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55,212-2. Brain Res 877: 407–410.
Lichtman AH, Dimen KR, Martin BR (1995). Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119: 282–290.
Mallet PE, Beninger RJ (1998). Delta-9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci 62: 2431–2434.
Marco EM, Perez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E et al (2004). Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav Pharmacol 15: 21–27.
McGregor IS, Issakidis CN, Prior G (1996). Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav 53: 657–664.
Meyers RA, Zavala AR, Speer CM, Neisewander JL (2006). Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120: 401–412.
Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS (2001). Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphetamine (‘ecstasy’). Eur J Pharmacol 433: 91–99.
Nakamura EM, da Silva EA, Concilio GV, Wilkinson DA, Masur J (1991). Reversible effects of acute and long-term administration of Δ-9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alcohol Depend 28: 167–175.
O'Brien E, Dedova I, Duffy L, Karl T, Matsumoto I (2006). Effects of chronic risperidone treatment on the striatal protein profiles in rats. Brain Res 1113: 24–32.
O'Shea M, McGregor IS, Mallet PE (2006). Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long-lasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol 20: 611–621.
O'Shea M, Singh ME, McGregor IS, Mallet PE (2004). Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol 18: 502–508.
Parker LA, Gillies T (1995). THC-induced place and taste aversions in Lewis and Sprague–Dawley rats. Behav Neurosci 109: 71–78.
Parmentier-Batteur S, Jin K, Xie L, Mao XO, Greenberg DA (2002). DNA microarray analysis of cannabinoid signaling in mouse brain in vivo. Mol Pharmacol 62: 828–835.
Philpot RM, Badanich KA, Kirstein CL (2003). Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res 27: 593–599.
Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella A-MG, Pennisi G et al (2006). Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging 27: 1020–1034.
Powell K (2006). Neurodevelopment: how does the teenage brain work? Nature 442: 865–867.
Price NE, Mumby MC (1999). Brain protein serine/threonine phosphatases. Curr Opin Neurobiol 9: 336–342.
Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G (2006). Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci 9: 1526–1533.
Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF et al (1996). Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther 276: 56–64.
Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M (2007). On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem 14: 36–46.
Sanudo-Pena MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM (1997). Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett 223: 125–128.
Sargent CA, Dunham I, Trowsdale J, Campbell RD (1989). Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci USA 86: 1968–1972.
Schneider M, Drews E, Koch M (2005). Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol 16: 447–454.
Schneider M, Koch M (2003). Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28: 1760–1769.
Sharifzadeh M, Haghighat A, Tahsili-Fahadan P, Khalaj S, Zarrindast MR, Zamanian AR (2006). Intra-hippocampal inhibition of protein kinase AII attenuates morphine-induced conditioned place preference. Pharmacol Biochem Behav 85: 705–712.
Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463.
Stiglick A, Kalant H (1985). Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology 85: 436–439.
Substance Abuse and Mental Health Services Administration (2005). The national survey on drug use and health report: age at first use of marijuana and past year serious mental illness. Office of Applied Statistics: Rockville, MD.
Tagliaferro P, Javier Ramos A, Onaivi ES, Evrard SG, Lujilde J, Brusco A (2006). Neuronal cytoskeleton and synaptic densities are altered after a chronic treatment with the cannabinoid receptor agonist WIN 55,212-2. Brain Res 1085: 163–176.
Tahir SK, Trogadis JE, Stevens JK, Zimmerman AM (1992). Cytoskeletal organization following cannabinoid treatment in undifferentiated and differentiated PC12 cells. Biochem Cell Biol 70: 1159–1173.
Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, Toda T, Sakaida I et al (2003). Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics 3: 2487–2493.
Tchaikovskaya T, Fraifeld V, Urphanishvili T, Andorfer JH, Davies P, Listowsky I (2005). Glutathione S-transferase hGSTM3 and ageing-associated neurodegeneration: relationship to Alzheimer's disease. Mech Ageing Dev 126: 309–315.
Valjent E, Maldonado R (2000). A behavioural model to reveal place preference to Delta 9-tetrahydrocannabinol in mice. Psychopharmacology 147: 436–438.
Vanrobaeys F, Van Coster R, Dhondt G, Devreese B, Van Beeumen J (2005). Profiling of myelin proteins by 2D-gel electrophoresis and multidimensional liquid chromatography coupled to MALDI TOF-TOF mass spectrometry. J Proteome Res 4: 2283–2293.
Viveros MP, Marco EM, File SE (2005). Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav 81: 331–342.
Wilmouth CE, Spear LP (2004). Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann NY Acad Sci 1021: 462–464.
Wilson Jr RG, Tahir SK, Mechoulam R, Zimmerman S, Zimmerman AM (1996). Cannabinoid enantiomer action on the cytoarchitecture. Cell Biol Int 20: 147–157.
Winer BJ, Brown DR, Michels KM (1991). Statistical Principles in Experimental Design. McGraw-Hill: New York.
Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA (2006). Two brain sites for cannabinoid reward. J Neurosci 26: 4901–4907.
Zatta P, Lain E, Cagnolini C (2000). Effects of aluminum on activity of Krebs cycle enzymes and glutamate dehydrogenase in rat brain homogenate. Eur J Biochem 267: 3049–3055.
Acknowledgements
This research was supported by an Australian Research Council grant to ISM and PEM and a National Health and Medical Research Council grant to ISM and JCA. We thank Ling Li for technical assistance and to Darek Figa and Debbie Brookes for animal care.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICTS OF INTEREST
The authors have no financial interest in the experiments described in the paper and their work has not been funded by any commercial company.
No authors have any compensation payments to claim.
Rights and permissions
About this article
Cite this article
Quinn, H., Matsumoto, I., Callaghan, P. et al. Adolescent Rats Find Repeated Δ9-THC Less Aversive Than Adult Rats but Display Greater Residual Cognitive Deficits and Changes in Hippocampal Protein Expression Following Exposure. Neuropsychopharmacol 33, 1113–1126 (2008). https://doi.org/10.1038/sj.npp.1301475
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301475
Keywords
This article is cited by
-
Cytotoxicity associated with acute and chronic administration of synthetic cannabinoids “Strox” in the brain, liver, heart, and testes of male albino rats: histological and immunohistochemical study
Egyptian Journal of Forensic Sciences (2023)
-
Adolescent Δ-9-tetrahydrocannabinol exposure induces differential acute and long-term neuronal and molecular disturbances in dorsal vs. ventral hippocampal subregions
Neuropsychopharmacology (2023)
-
Long-term effects of THC exposure on reward learning and motivated behavior in adolescent and adult male rats
Psychopharmacology (2023)
-
Cellular messenger molecules mediating addictive drug-induced cognitive impairment: cannabinoids, ketamine, methamphetamine, and cocaine
Future Journal of Pharmaceutical Sciences (2022)
-
Adolescents are more sensitive than adults to acute behavioral and cognitive effects of THC
Neuropsychopharmacology (2022)