Abstract
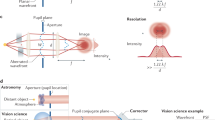
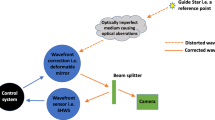
High-resolution in vivo imaging is of great importance for the fields of biology and medicine. The introduction of hardware-based adaptive optics (HAO) has pushed the limits of optical imaging, enabling high-resolution near diffraction-limited imaging of previously unresolvable structures1,2. In ophthalmology, when combined with optical coherence tomography, HAO has enabled a detailed three-dimensional visualization of photoreceptor distributions3,4 and individual nerve fibre bundles5 in the living human retina. However, the introduction of HAO hardware and supporting software adds considerable complexity and cost to an imaging system, limiting the number of researchers and medical professionals who could benefit from the technology. Here we demonstrate a fully automated computational approach that enables high-resolution in vivo ophthalmic imaging without the need for HAO. The results demonstrate that computational methods in coherent microscopy are applicable in highly dynamic living systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
10 September 2015
The authors acknowledge that two highly relevant manuscripts should have been cited in this Letter: Meitav, N. & Ribak, E. N. Improving retinal image resolution with iterative weighted shift-and-add. J. Opt. Soc. Am. A 28, 1395–1402 (2011) Meitav, N. & Ribak, E. N. Estimation of the ocular point spread function by retina modeling. Opt Lett. 37, 1466–1468 (2012) These manuscripts report progress towards in vivo high-resolution retinal imaging without using hardware-based adaptive optics by averaging out high-order, temporally changing aberrations, and by applying various image filters to the intensity of backscattered optical signals..
References
Liang, J., Williams, D. R. & Miller, D. T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A 14, 2884–2892 (1997).
Hermann, B. et al. Adaptive-optics ultrahigh-resolution optical coherence tomography. Opt. Express 29, 2142–2144 (2004).
Zhang, Y. et al. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Opt. Express 14, 4380–4394 (2006).
Felberer, F. et al. Adaptive optics SLO/OCT for 3D imaging of human photoreceptors in vivo. Biomed. Opt. Express 5, 439–456 (2014).
Kocaoglu, O. P. et al. Imaging retinal nerve fiber bundles using optical coherence tomography with adaptive optics. Vision Res. 51, 1835–1844 (2011).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Drexler, W. & Fujimoto, J. G. Optical Coherence Tomography: Technology and Applications (Springer, 2008).
Sánchez-Tocino, H. et al. Retinal thickness study with optical coherence tomography in patients with diabetes. Invest. Ophthalmol. Vis. Sci. 43, 1588–1594 (2002).
Saidha, S. et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 134, 518–533 (2011).
Iglesias, I. & Artal, P. High-resolution retinal images obtained by deconvolution from wave-front sensing. Opt. Lett. 25, 1804–1806 (2000).
Christou, J. C., Roorda, A. & Williams, D. R. Deconvolution of adaptive optics retinal images. J. Opt. Soc. Am. A 21, 1393–1401 (2004).
Ralston, T. S., Marks, D. L., Carney, P. S. & Boppart, S. A. Interferometric synthetic aperture microscopy. Nature Phys. 3, 129–134 (2007).
Adie, S. G., Graf, B. W., Ahmad, A., Carney, P. S. & Boppart, S. A. Computational adaptive optics for broadband optical interferometric tomography of biological tissue. Proc. Natl Acad. Sci. USA 109, 7175–7180 (2012).
Kumar, A., Drexler, W. & Leitgeb, R. A. Subaperture correlation based digital adaptive optics for full field optical coherence tomography. Opt. Express 21, 10850–10866 (2013).
Adie, S. G. et al. Guide-star-based computational adaptive optics for broadband interferometric tomography. Appl. Phys. Lett. 101, 221117 (2012).
Shemonski, N. D. et al. Stability in computed optical interferometric tomography (part I): stability requirements. Opt. Express 22, 19183–19197 (2014).
Ralston, T. S., Marks, D. L., Carney, P. S. & Boppart, S. A. Phase stability technique for inverse scattering in optical coherence tomography. Proc. 3rd IEEE Int. Symp. on Biomed. Imaging: Nano to Macro 578–581 (2006).
Ahmad, A. et al. Real-time in vivo computed optical interferometric tomography. Nature Photon. 7, 444–448 (2013).
Shemonski, N. D. et al. Stability in computed optical interferometric tomography (part II): in vivo stability assessment. Opt. Express 22, 19314–19326 (2014).
Liu, Y.-Z. et al. Computed optical interferometric tomography for high-speed volumetric cellular imaging. Biomed. Opt. Express 5, 2988–3000 (2014).
Martinez-Conde, S., Macknik, S. L. & Hubel, D. H. The role of fixational eye movements in visual perception. Nature Rev. Neurosci. 5, 229–240 (2004).
Curcio, C. A., Sloan, K. R., Kalina, R. E. & Hendrickson, A. E. Human photoreceptor topography. J. Comp. Neurol. 292, 497–523 (1990).
Yadlowsky, M. J., Schmitt, J. M. & Bonner, R. F. Multiple scattering in optical coherence microscopy. Appl. Opt. 34, 5699–5707 (1995).
Castejon-Mochon, J. F., Lopez-Gil, N., Benito, A. & Artal, P. Ocular wave-front aberration statistics in a normal young population. Vision Res. 42, 1611–1617 (2002).
Acknowledgements
The authors thank D. Spillman and E. Chaney from the Beckman Institute for Advanced Science and Technology for their assistance with operations and human study protocol support, respectively. This research was supported in part by grants from the National Institutes of Health (NIBIB, 1 R01 EB013723, 1 R01 EB012479) and the National Science Foundation (CBET 14-45111).
Author information
Authors and Affiliations
Contributions
N.D.S. constructed the optical system, collected data, analysed data and wrote the paper. F.A.S., Y-Z.L, and S.G.A. collected and analysed data, and assisted in writing the paper. P.S.C. contributed the theoretical and mathematical basis for these methods, reviewed and edited the manuscript, and helped obtain funding. S.A.B. conceived of the study, analysed the data, reviewed and edited the manuscript and helped obtain funding.
Corresponding author
Ethics declarations
Competing interests
S.A.B. and P.S.C. are co-founders of Diagnostic Photonics, which is licensing intellectual property from the University of Illinois at Urbana-Champaign related to interferometric synthetic aperture microscopy. S.A.B. also receives royalties from the Massachusetts Institute of Technology for patents related to optical coherence tomography. S.G.A., P.S.C. and S.A.B. are listed as inventors on a patent application (application no. 20140050382) related to the work presented in this manuscript. All other authors have nothing to disclose.
Supplementary information
Supplementary information
10.1038/nrrheum.2015.85 (PDF 1540 kb)
Rights and permissions
About this article
Cite this article
Shemonski, N., South, F., Liu, YZ. et al. Computational high-resolution optical imaging of the living human retina. Nature Photon 9, 440–443 (2015). https://doi.org/10.1038/nphoton.2015.102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2015.102
This article is cited by
-
Label-free biomedical optical imaging
Nature Photonics (2023)
-
Mapping nanoscale topographic features in thick tissues with speckle diffraction tomography
Light: Science & Applications (2023)
-
Label-free metabolic and structural profiling of dynamic biological samples using multimodal optical microscopy with sensorless adaptive optics
Scientific Reports (2022)
-
Resolution-enhanced OCT and expanded framework of information capacity and resolution in coherent imaging
Scientific Reports (2021)
-
Cellular-resolution in vivo tomography in turbid tissue through digital aberration correction
PhotoniX (2020)