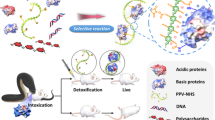
Abstract
Detoxification treatments such as toxin-targeted anti-virulence therapy1,2 offer ways to cleanse the body of virulence factors that are caused by bacterial infections, venomous injuries and biological weaponry. Because existing detoxification platforms such as antisera3, monoclonal antibodies4, small-molecule inhibitors5,6 and molecularly imprinted polymers7 act by targeting the molecular structures of toxins, customized treatments are required for different diseases. Here, we show a biomimetic toxin nanosponge that functions as a toxin decoy in vivo. The nanosponge, which consists of a polymeric nanoparticle core surrounded by red blood cell membranes, absorbs membrane-damaging toxins and diverts them away from their cellular targets. In a mouse model, the nanosponges markedly reduce the toxicity of staphylococcal alpha-haemolysin (α-toxin) and thus improve the survival rate of toxin-challenged mice. This biologically inspired toxin nanosponge presents a detoxification treatment that can potentially treat a variety of injuries and diseases caused by pore-forming toxins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clatworthy, A. E., Pierson, E. & Hung, D. T. Targeting virulence: a new paradigm for antimicrobial therapy. Nature Chem. Biol. 3, 541–548 (2007).
Rasko, D. A. & Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nature Rev. Drug Discov. 9, 117–128 (2010).
Beghini, D. G. et al. Anti-sera raised in rabbits against crotoxin and phospholipase A2 from Crotalus durissus cascavella venom neutralize the neurotoxicity of the venom and crotoxin. Toxicon 44, 141–148 (2004).
Chen, Z. et al. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc. Natl Acad. Sci. USA 106, 13487–13492 (2009).
McCormick, C. C., Caballero, A. R., Balzli, C. L., Tang, A. & O'Callaghan, R. J. Chemical inhibition of alpha-toxin, a key corneal virulence factor of Staphylococcus aureus. Invest. Ophthalmol. Vis. Sci. 50, 2848–2854 (2009).
Hung, D. T., Shakhnovich, E. A., Pierson, E. & Mekalanos, J. J. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310, 670–674 (2005).
Hoshino, Y. et al. The rational design of a synthetic polymer nanoparticle that neutralizes a toxic peptide in vivo. Proc. Natl Acad. Sci. USA 109, 33–38 (2012).
Gilbert, R. J. Pore-forming toxins. Cell Mol. Life Sci. 59, 832–844 (2002).
Rosado, C. J. et al. The MACPF/CDC family of pore-forming toxins. Cell Microbiol. 10, 1765–1774 (2008).
Shoham, M. Antivirulence agents against MRSA. Future Med. Chem. 3, 775–777 (2011).
O'Hanley, P., Lalonde, G. & Ji, G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect. Immun. 59, 1153–1161 (1991).
Edelson, B. T. & Unanue, E. R. Intracellular antibody neutralizes Listeria growth. Immunity 14, 503–512 (2001).
Nakouzi, A., Rivera, J., Rest, R. F. & Casadevall, A. Passive administration of monoclonal antibodies to anthrolysin O prolong survival in mice lethally infected with Bacillus anthracis. BMC Microbiol. 8, 159–168 (2008).
Kirkham, L. A. et al. Construction and immunological characterization of a novel nontoxic protective pneumolysin mutant for use in future pneumococcal vaccines. Infect. Immun. 74, 586–593 (2006).
Andreeva-Kovalevskaya, Zh. I., Solonin, A. S., Sineva, E. V. & Ternovsky, V. I. Pore-forming proteins and adaptation of living organisms to environmental conditions. Biochemistry (Mosc) 73, 1473–1492 (2008).
Bayley, H. Membrane–protein structure: piercing insights. Nature 459, 651–652 (2009).
Hu, C. M. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Bhakdi, S., Tranum-Jensen, J. & Sziegoleit, A. Mechanism of membrane damage by streptolysin-O. Infect. Immun. 47, 52–60 (1985).
Dempsey, C. E. The actions of melittin on membranes. Biochim. Biophys. Acta 1031, 143–161 (1990).
Klainer, A. S., Madoff, M. A., Cooper, L. Z. & Weinstein, L. Staphylococcal alpha-hemolysin: detection on the erythrocyte membrane by immunofluorescence. Science 145, 714–715 (1964).
Moorjani, M. et al. Nanoerythrosomes, a new derivative of erythrocyte ghost II: identification of the mechanism of action. Anticancer Res. 16, 2831–2836 (1996).
Gao, W., Langer, R. & Farokhzad, O. C. Poly(ethylene glycol) with observable shedding. Angew. Chem. Int. Ed. 49, 6567–6571 (2010).
Bhakdi, S. & Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 55, 733–751 (1991).
Gill, D. M. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46, 86–94 (1982).
Watanabe, M., Tomita, T. & Yasuda, T. Membrane-damaging action of staphylococcal alpha-toxin on phospholipid-cholesterol liposomes. Biochim. Biophys. Acta 898, 257–265 (1987).
Pornpattananangkul, D. et al. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J. Am. Chem. Soc. 133, 4132–4139 (2011).
Leroux, J. C. Injectable nanocarriers for biodetoxification. Nature Nanotech. 2, 679–684 (2007).
Acknowledgements
This work is supported by the National Science Foundation (grant no. DMR-1216461) and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (award no. R01DK095168). B.L. is supported by a National Institutes of Health training grant (R25CA153915 ) from the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
L.Z. conceived and designed the experiments with C-M.H., R.F. and J.C. C-M.H., R.F., J.C. and B.L. performed all the experiments. All authors analysed and discussed the data. L.Z., C-M.H. and R.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1933 kb)
Rights and permissions
About this article
Cite this article
Hu, CM., Fang, R., Copp, J. et al. A biomimetic nanosponge that absorbs pore-forming toxins. Nature Nanotech 8, 336–340 (2013). https://doi.org/10.1038/nnano.2013.54
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2013.54
This article is cited by
-
Synergy and Coordination Between Biomimetic Nanoparticles and Biological Cells/Tissues/Organs/Systems: Applications in Nanomedicine and Prospect
Biomedical Materials & Devices (2024)
-
Systematic evaluation of membrane-camouflaged nanoparticles in neutralizing Clostridium perfringens ε-toxin
Journal of Nanobiotechnology (2023)
-
Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment
Nature Communications (2023)
-
A smart pathogen detector engineered from intracellular hydrogelation of DNA-decorated macrophages
Nature Communications (2023)
-
Macrophage membrane-derived pH-responsive nanovesicles to target tumor cells with integrin α4β1 receptor
Macromolecular Research (2023)