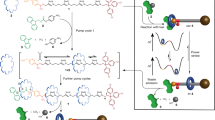
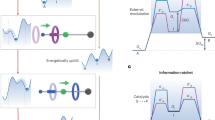
Abstract
Carrier proteins consume fuel in order to pump ions or molecules across cell membranes, creating concentration gradients. Their control over diffusion pathways, effected entirely through noncovalent bonding interactions, has inspired chemists to devise artificial systems that mimic their function. Here, we report a wholly artificial compound that acts on small molecules to create a gradient in their local concentration. It does so by using redox energy and precisely organized noncovalent bonding interactions to pump positively charged rings from solution and ensnare them around an oligomethylene chain, as part of a kinetically trapped entanglement. A redox-active viologen unit at the heart of a dumbbell-shaped molecular pump plays a dual role, first attracting and then repelling the rings during redox cycling, thereby enacting a flashing energy ratchet mechanism with a minimalistic design. Our artificial molecular pump performs work repetitively for two cycles of operation and drives rings away from equilibrium toward a higher local concentration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vinothkumar, K. R. & Henderson, R. Structures of membrane proteins. Quart. Rev. Biophys. 43, 65–158 (2010).
Boyer, P. D. Energy, life, and ATP (Nobel lecture). Angew. Chem. Int. Ed. 37, 2296–2307 (1998).
Walker, J. E. ATP synthesis by rotary catalysis (Nobel lecture). Angew. Chem. Int. Ed. 37, 2308–2319 (1998).
Skou, J. C. The identification of the sodium–potassium pump (Nobel lecture). Angew. Chem. Int. Ed. 37, 2320–2328 (1998).
Shi, Y. G. Common folds and transport mechanisms of secondary active transporters. Annu. Rev. Biophys. 42, 51–72 (2013).
Gai, F., Hasson, K. C., McDonald, J. C. & Anfinrud, P. A. Chemical dynamics in proteins: the photoisomerization of retinal in bacteriorhodopsin. Science 279, 1886–1891 (1998).
Brandt, U. Energy converting NADH: quinone oxidoreductase (complex I). Annu. Rev. Biochem. 75, 69–92 (2006).
Sazanoz, L. A. The mechanism of coupling between electron transfer and proton translocation in respiratory complex I. J. Bioenerg. Biomembr. 46, 247–253 (2014).
Astumian, R. D. Microscopic reversibility as the organizing principle of molecular machines. Nature Nanotech. 7, 684–688 (2012).
Kinbara, K. & Aida, T. Toward intelligent molecular machines: directed motions of biological and artificial molecules and assemblies. Chem. Rev. 105, 1377–1400 (2005).
Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007).
Krishnan, Y. & Simmel, F. C. Nucleic acid based molecular devices. Angew. Chem. Int. Ed. 50, 3124–3156 (2011).
Coskun, A., Banaszak, M., Astumian, R. D., Stoddart, J. F. & Grzybowski, B. A. Great expectations: can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 41, 19–30 (2012).
Michl, J. & Sykes, E. C. H. Molecular rotors and motors: recent advances and future challenges. ACS Nano 3, 1042–1048 (2009).
Vogelsberg, C. S. & Garcia-Garibay, M. A. Crystalline molecular machines: function, phase order, dimensionality, and composition. Chem. Soc. Rev. 41, 1892–1910 (2012).
Steinburgh-Yfrach, G. et al. Conversion of light energy to proton potential in liposomes by artificial photosynthetic reaction centres. Nature 385, 239–241 (1997).
Bhosale, S. et al. Photoproduction of proton gradients with π-stacked fluorophore scaffolds in lipid bilayers. Science 313, 84–86 (2006).
Xie, X., Crespo, G. A., Mistlberger, G. & Bakker, E. Photocurrent generation based on a light-driven proton pump in an artificial liquid membrane. Nature Chem. 6, 202–207 (2014).
Bennett, I. M. et al. Active transport of Ca2+ by an artificial photosynthetic membrane. Nature 420, 398–401 (2002).
Li, Q. et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nature Nanotech. 10, 161–165 (2015).
Bruns, C. & Stoddart, J. F. Rotaxane-based molecular muscles. Acc. Chem. Res. 47, 2186–2199 (2014).
Muraoka, T., Kinbara, K. & Aida, T. Mechanical twisting of a guest by a photoresponsive host. Nature 440, 512–515 (2006).
Brown, R. A., Diemer, V., Webb, S. J. & Clayden, J. End-to-end conformational communication through a synthetic purinergic receptor by ligand-induced helicity switching. Nature Chem. 5, 853–860 (2013).
Leigh, D. A., Lewandowska, U., Lewandowski, B. & Wilson, M. R. Synthetic molecular walkers. Top. Curr. Chem. 354, 111–138 (2014).
Fletcher, S. P., Dumur, F., Pollard, M. M. & Feringa, B. L. A reversible, unidirectional molecular rotary motor driven by chemical energy. Science 310, 80–82 (2005).
Lu, C.-H., Cecconello, A., Elbaz, J., Credi, A. & Willner, I. A three-station DNA catenane rotary motor with controlled directionality. Nano Lett. 13, 2303–2308 (2013).
Greb, L. & Lehn, J-M. Light-driven molecular motors: imines as four-step or two-step unidirectional rotors. J. Am. Chem. Soc. 136, 13114–13117 (2014).
Chatterjee, M. N., Kay, E. R. & Leigh, D. A. Beyond switches: ratcheting a particle energetically uphill with a compartmentalized molecular machine. J. Am. Chem. Soc. 128, 4058–4073 (2006).
Serreli, V., Lee, C-F., Kay, E. R. & Leigh, D. A. A molecular information ratchet. Nature 445, 523–527 (2007).
Van Dongen, S. F. M., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Processive catalysis. Angew. Chem. Int. Ed. 53, 11420–11428 (2014).
Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013).
Yoon, H. J., Kuwabara, J., Kim, J-H. & Mirkin, C. A. Allosteric supramolecular triple-layer catalysts. Science 330, 66–69 (2010).
Wang, J. & Feringa, B. L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. Science 331, 1429–1432 (2011).
He, Y. & Liu, D. Autonomous multistep organic synthesis in a single isothermal solution mediated by a DNA walker. Nature Nanotech. 5, 778–782 (2010).
McKee, M. L. et al. Programmable one-pot multistep organic synthesis using DNA junctions. J. Am. Chem. Soc. 134, 1446–1449 (2012).
Gu, H., Chao, J., Xiao, S-J. & Seeman, N. C. A proximity-based programmable DNA nanoscale assembly line. Nature 465, 202–205 (2010).
Ashton, P. R. et al. A [2]catenane made to order. Angew. Chem. Int. Ed. Engl. 28, 1396–1399 (1989).
Collier, C. P. et al. A [2]catenane-based solid state electronically reconfigurable switch. Science 289, 1172–1175 (2000).
Odell, B. et al. Cyclobis(paraquat-p-phenylene): a tetracationic multipurpose receptor. Angew. Chem. Int. Ed. Engl. 27, 1547–1550 (1988).
Trabolsi, A. et al. Radically enhanced molecular recognition. Nature Chem. 2, 42–49 (2010).
Fahrenbach, A. C. et al. Solution-phase mechanistic study and solid-state structure of a tris(bipyridinium radical cation) inclusion complex. J. Am. Chem. Soc. 134, 3061–3072 (2012).
Cheng, C. et al. Energetically demanding transport in a supramolecular assembly. J. Am. Chem. Soc. 136, 14702–14705 (2014).
Li, H. et al. Relative unidirectional translation in an artificial molecular assembly fueled by light. J. Am. Chem. Soc. 135, 18609–18620 (2013).
McGonigal, P. R. et al. Controlling association kinetics in the formation of donor–acceptor pseudorotaxanes. Tetrahedron Lett. http://dx.doi.org/10.1016/j.tetlet.2015.01.169 (2015)
Sevick, E. M. & Williams, D. R. M. A piston-rotaxane with two potential stripes: force transitions and yield stresses. Molecules 18, 13398–13409 (2013).
Lehn, J-M. Supramolecular Chemistry: Concepts and Perspectives (Wiley-VCH, 1995).
Ragazzon, G., Baroncini, M., Silvi, S., Venturi, M. & Credi, A. Light-powered autonomous and directional molecular motion of a dissipative self-assembling system. Nature Nanotech. 10, 70–75 (2015).
Acknowledgements
This material is based on work supported by the National Science Foundation (NSF; CHE-1308107). The authors acknowledge the Integrated Molecular Structure Education and Research Center at Northwestern University for providing access to equipment for relevant experiments. The authors acknowledge the QUEST High-Performance Computing Cluster at Northwestern University for a research allocation of computer time. S.T.S. thanks the International Institute for Nanotechnology (IIN) at Northwestern University for a postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
C.C. and N.A.V. conceived the project. C.C. designed, synthesized and tested the compounds. P.R.M., S.T.S. and H.L. performed the barrier searching work. P.R.M. and C.C. wrote the paper. N.A.V. and C.K. helped in evaluating the results and commented on the contents of the manuscript. J.F.S. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5711 kb)
Rights and permissions
About this article
Cite this article
Cheng, C., McGonigal, P., Schneebeli, S. et al. An artificial molecular pump. Nature Nanotech 10, 547–553 (2015). https://doi.org/10.1038/nnano.2015.96
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2015.96
This article is cited by
-
Photo-responsive functional materials based on light-driven molecular motors
Light: Science & Applications (2024)
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)
-
Using supramolecular machinery to engineer directional charge propagation in photoelectrochemical devices
Nature Chemistry (2023)
-
An electric molecular motor
Nature (2023)
-
Iontronic components: From liquid- to solid-states
Nano Research (2023)