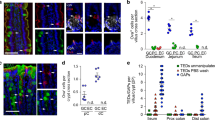
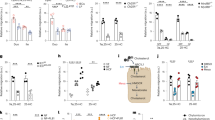
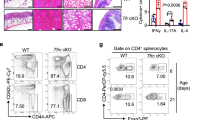
Abstract
In humans and other mammals it is known that calcium and phosphate ions are secreted from the distal small intestine into the lumen. However, why this secretion occurs is unclear. Here, we show that the process leads to the formation of amorphous magnesium-substituted calcium phosphate nanoparticles that trap soluble macromolecules, such as bacterial peptidoglycan and orally fed protein antigens, in the lumen and transport them to immune cells of the intestinal tissue. The macromolecule-containing nanoparticles utilize epithelial M cells to enter Peyer's patches, small areas of the intestine concentrated with particle-scavenging immune cells. In wild-type mice, intestinal immune cells containing these naturally formed nanoparticles expressed the immune tolerance-associated molecule ‘programmed death-ligand 1’, whereas in NOD1/2 double knockout mice, which cannot recognize peptidoglycan, programmed death-ligand 1 was undetected. Our results explain a role for constitutively formed calcium phosphate nanoparticles in the gut lumen and show how this helps to shape intestinal immune homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
17 March 2015
In the version of this Article originally published online, in the Methods, in the first sentence of the section 'Nuclear microscopy', ref. 7 should have been ref. 2, and in the Reference list ref. 6 was cited out of order; it should have been ref. 31. These errors have now been corrected in all versions of the Article and the references have been re-numbered.
References
Powell, J. J., Thoree, V. & Pele, L. C. Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract. Br. J. Nutr. 98(Suppl 1), S59–S63 (2007).
Gomez-Morilla, I., Thoree, V., Powell, J. J., Kirkby, K. J. & Grime, G. W. Identification and quantitative analysis of calcium phosphate microparticles in intestinal tissue by nuclear microscopy. Nucl. Instrum. Methods Phys. Res. B 249, 665–669 (2006).
Powell, J. J., Faria, N., Thomas-McKay, E. & Pele, L. C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 34, J226–J233 (2010).
Lentner, C. (ed.) Geigy Scientific Tables 8th edn, Vol. 1 (CIBA-GEIGY Ltd, 1981).
Schedl, H. P., Osbaldiston, G. W. & Mills, I. H. Absorption, secretion, and precipitation of calcium in the small intestine of the dog. Am J Physiol 214, 814–819 (1968).
Jung, C., Hugot, J. P. & Barreau, F. Peyer's patches: the immune sensors of the intestine. Int. J. Inflam. 2010, 823710 (2010).
Mowat, A. M. & Bain, C. C. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 3, 550–564 (2011).
Pele, L. C. et al. Low dietary calcium levels modulate mucosal caspase expression and increase disease activity in mice with dextran sulfate sodium induced colitis. J. Nutr. 137, 2475–2480 (2007).
Civitelli, R. & Ziambaras, K. Calcium and phosphate homeostasis: concerted interplay of new regulators. J. Endocrinol. Invest. 34, 3–7 (2011).
Neutra, M. R. & Kraehenbuhl, J. P. Transepithelial transport and mucosal defence I: the role of M cells. Trends Cell Biol. 2, 134–138 (1992).
Knoop, K. A. et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J. Immunol. 183, 5738–5747 (2009).
Kimura, S. et al. Visualization of the entire differentiation process of murine M cells: suppression of their maturation in cecal patches. Mucosal Immunol. http://www.nature.com/mi/journal/vaop/ncurrent/full/mi201499a.html (2014).
Masahata, K. et al. Generation of colonic IgA-secreting cells in the caecal patch. Nature Commun. 5, 3704 (2014).
Szentkuti, L. Light microscopical observations on luminally administered dyes, dextrans, nanospheres and microspheres in the pre-epithelial mucus gel layer of the rat distal colon. J. Control. Rel. 46, 233–242 (1997).
Uskokovic, V. & Uskokovic, D. P. Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res B 96, 152–191 (2011).
Dorozhkin, S. V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 6, 715–734 (2010).
Blander, J. M. & Medzhitov, R. Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 (2004).
Blander, J. M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).
Chong, C. S. et al. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J. Control. Rel. 102, 85–99 (2005).
Heit, A., Schmitz, F., Haas, T., Busch, D. H. & Wagner, H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur. J. Immunol. 37, 2063–2074 (2007).
Schlosser, E. et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine 26, 1626–1637 (2008).
Klasen, I. S. et al. The presence of peptidoglycan–polysaccharide complexes in the bowel wall and the cellular responses to these complexes in Crohn's disease. Clin. Immunol. Immunopathol. 71, 303–308 (1994).
Schrijver, I. A. et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain 124, 1544–1554 (2001).
Boskey, A. L. & Posner, A. S. Magnesium stabilization of amorphous calcium phosphate: a kinetic study. Mater. Res. Bull. 9, 907–916 (1974).
Termine, J. D., Peckauskas, R. A. & Posner, A. S. Calcium phosphate formation in vitro. II. Effects of environment on amorphous-crystalline transformation. Arch. Biochem. Biophys. 140, 318–325 (1970).
Hewitt, R. E. et al. Immuno-inhibitory PD-L1 can be induced by a peptidoglycan/NOD2 mediated pathway in primary monocytic cells and is deficient in Crohn's patients with homozygous NOD2 mutations. Clin. Immunol. 143, 162–169 (2012).
Davies, J. M., MacSharry, J. & Shanahan, F. Differential regulation of Toll-like receptor signalling in spleen and Peyer's patch dendritic cells. Immunology 131, 438–448 (2010).
Stroo, I. et al. Phenotyping of Nod1/2 double deficient mice and characterization of Nod1/2 in systemic inflammation and associated renal disease. Biol. Open. 1, 1239–1247 (2012).
Francisco, L. M., Sage, P. T. & Sharpe, A. H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242 (2010).
Van Heel, D. A. et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet 365, 1794–1796 (2005).
Gullberg, E. & Soderholm, J. D. Peyer's patches and M cells as potential sites of the inflammatory onset in Crohn's disease. Ann. NY Acad. Sci. 1072, 218–232 (2006).
Fukaya, T. et al. Crucial roles of B7-H1 and B7-DC expressed on mesenteric lymph node dendritic cells in the generation of antigen-specific CD4+Foxp3+ regulatory T cells in the establishment of oral tolerance. Blood 116, 2266–2276 (2010).
Scandiuzzi, L. et al. Tissue-expressed B7-H1 critically controls intestinal inflammation. Cell. Rep. 6, 625–632 (2014).
Reynoso, E. D. et al. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J. Immunol. 182, 2102–2112 (2009).
Awaad, A., Nakamura, M. & Ishimura, K. Imaging of size-dependent uptake and identification of novel pathways in mouse Peyer's patches using fluorescent organosilica particles. Nanomedicine 8, 627–636 (2012).
Sass, W., Dreyer, H. P. & Seifert, J. Rapid insorption of small particles in the gut. Am. J. Gastroenterol. 85, 255–260 (1990).
Davies, K. M., Rafferty, K. & Heaney, R. P. Determinants of endogenous calcium entry into the gut. Am. J. Clin. Nutr. 80, 919–923 (2004).
Cross, K. J., Huq, N. L., Palamara, J. E., Perich, J. W. & Reynolds, E. C. Physicochemical characterization of casein phosphopeptide–amorphous calcium phosphate nanocomplexes. J. Biol. Chem. 280, 15362–15369 (2005).
De Kruif, C. G., Huppertz, T., Urban, V. S. & Petukhov, A. V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 171–172, 36–52 (2012).
McGann, T. C. et al. Amorphous calcium phosphate in casein micelles of bovine milk. Calcif. Tissue Int. 35, 821–823 (1983).
Minniti, F. et al. Breast-milk characteristics protecting against allergy. Endocr. Metab. Immune Disord. Drug Targets 14, 9–15 (2014).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675 (2012).
Bilton, M., Brown, A. P. & Milne, S. J. Investigating the optimum conditions for the formation of calcium oxide, used for CO2 sequestration, by thermal decomposition of calcium acetate. Electron Microscopy and Analysis Group Conference 2011 (Emag 2011) 371 ( 2012).
Bres, E. F., Hutchison, J. L., Senger, B., Voegel, J. C. & Frank, R. M. HREM study of irradiation damage in human dental enamel crystals. Ultramicroscopy 35, 305–322 (1991).
Acknowledgements
The authors thank the UK Medical Research Council (grant no. U105960399) for their continued support and the UK Engineering and Physical Sciences Research Council for support of the Ion Beam Centre as a UK National Facility (GR/R50097). The authors acknowledge the Dairy Council and the Sir Halley Stewart Trust for support. Work in the laboratory of J.D.L is supported by the Dutch MS Research Foundation. The authors thank M-J. Melief for help in modifying in situ techniques for the detection of peptidoglycan and I. Stroo for providing snap-frozen NOD1/2−/− samples from mice originally from S.E.G.'s laboratory. The authors thank J. Kaufman, A. Wyllie and P. Mastroeni (all University of Cambridge) for brainstorming and advice. J.C.H-G. and P.A.M. acknowledge financial support from the European Union's Seventh Framework Programme under a contract for an Integrated Infrastructure Initiative (reference no. 312483-ESTEEM2). P.A.M. also acknowledges financial support from the European Research Council (reference 291,522 3DIMAGE). N.A.M. and D.S.D. were supported by projects BB/J014762/1 and BB/K021257/1 and institute strategic programme grant funding from the Biological and Biotechnological Research Council. I.R.W. and D.R. were supported by grants from the National Institutes of Health (DK064730 and AI111388).
Author information
Authors and Affiliations
Contributions
J.J.P. developed the overall hypothesis and led the work and, with L.C.P., was involved in specific study design, data interpretation and writing of the paper. I.G-M., G.W.G. and K.J.K. developed the necessary techniques for, and undertook with V.T. and J.J.P., PIXE/nuclear microscopy studies. J.R. and V.T. developed and optimised immunostaining and carried out cell phenotyping for the confocal studies, which were carried out with J.N.S., who also prepared the samples for HAADF STEM imaging. E.T-M., J.C.H-G. and P.A.M. were responsible for the HAADF STEM tomography. E.T-M. assisted A.B. in undertaking TEM/STEM analyses, while V.T. and E.T-M. worked with J.N.S. to carry out SEM analyses. R.L. and R.P.H.T. provided mentorship in the early development of the work/hypothesis. G.L. and Y.T. undertook the initial feeding study with labelled OVA. S.E.G. developed and characterised NOD1/2−/− mice and supported sample analysis of their Peyer's patches. N.A.M., D.S.D., I.R.W. and D.R. designed and undertook the studies to determine the impact of M-cell deficiency on AMCP uptake. J.D.L. provided the antibody and expertise for 2E9 staining and interpretation and, with A.B., S.F.A.B., R.E.H. and C.T.H., provided rigorous data review and discussion with J.J.P. and L.C.P. All authors contributed to data interpretation and to the writing and critical review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 431 kb)
Supplementary information
Supplementary Movie 1 (AVI 48685 kb)
Supplementary information
Supplementary Movie 2 (MPG 7407 kb)
Rights and permissions
About this article
Cite this article
Powell, J., Thomas-McKay, E., Thoree, V. et al. An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells. Nature Nanotech 10, 361–369 (2015). https://doi.org/10.1038/nnano.2015.19
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2015.19
This article is cited by
-
Smarcad1 mediates microbiota-induced inflammation in mouse and coordinates gene expression in the intestinal epithelium
Genome Biology (2020)
-
Critical review of the safety assessment of titanium dioxide additives in food
Journal of Nanobiotechnology (2018)
-
Comprehensive organic profiling of biological particles derived from blood
Scientific Reports (2018)
-
Mouse Cre Models for the Study of Bone Diseases
Current Osteoporosis Reports (2018)
-
The unrecognized occupational relevance of the interaction between engineered nanomaterials and the gastro-intestinal tract: a consensus paper from a multidisciplinary working group
Particle and Fibre Toxicology (2017)