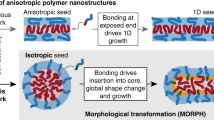
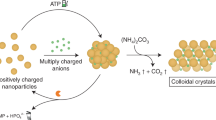
Abstract
The past 20 years have witnessed simultaneous multidisciplinary explosions in experimental techniques for synthesizing new materials, measuring and manipulating nanoscale structures, understanding biological processes at the nanoscale, and carrying out large-scale computations of many-atom and complex macromolecular systems. These advances have led to the new disciplines of nanoscience and nanoengineering. For reasons that are discussed here, most nanoparticles do not 'self-assemble' into their thermodynamically lowest energy state, and require an input of energy or external forces to 'direct' them into particular structures or assemblies. We discuss why and how a combination of self- and directed-assembly processes, involving interparticle and externally applied forces, can be applied to produce desired nanostructured materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933–937 (1996).
Hodes, G. When small is different: Some recent advances in concepts and applications of nanoscale phenomena. Adv. Mater. 19, 639–655 (2007).
Klabunde, K. J. S. (ed.) Nanoscale Materials in Chemistry (Wiley Interscience, New York, 2001).
Antonets, I. V., Kotov, L. N., Nekipelov, S. V. & Karpushov, E. N. Conducting and reflecting properties of thin metal films. Tech. Phys. 49, 1496–1500 (2004).
Lenham, A. P. & Treherne, D. M. Applicability of anomalous skin-effect theory to optical constants of Cu, Ag, and Au in the Infrared. J. Opt. Soc. Am. 56, 683–685 (1966).
Srivastava, D., Menon, M. & Cho, K. Anisotropic nanomechanics of boron nitride nanotubes: Nanostructured “skin” effect. Phys. Rev. B 6319, 195413 (2001).
Ercolessi, F., Andreoni, W. & Tosatti, E. Melting of small gold particles: Mechanism and size effects. Phys. Rev. Lett. 66, 911–914 (1991).
Hagen, M. H. J., Meijer, E. J., Mooij, G., Frenkel, D. & Lekkerkerker, H. N. W. Does C60 have a liquid-phase. Nature 365, 425–426 (1993).
Rey, C. & Gallego, L. J. Structures of hard-core Yukawa clusters and the tail-range dependence of the existence of a liquidlike cluster phase: Relevance to the physics of C-60. Phys. Rev. E 53, 2480–2487 (1996).
Israelachvili, J. Self-assembly in 2 dimensions — surface micelles and domain formation in monolayers. Langmuir 10, 3774–3781 (1994).
Israelachvili, J. N. Intermolecular and Surface Forces 2nd edn (Academic Press, Burlington, 1991).
Alcantar, N. A., Park, C., Pan, J. M. & Israelachvili, J. N. Adhesion and coalescence of ductile metal surfaces and nanoparticles. Acta Mater. 51, 31–47 (2003).
Rosensweig, R. E. Ferrohydrodynamics (Cambrige Univ. Press, New York, 1985).
Cho, K. S., Talapin, D. V., Gaschler, W. & Murray, C. B. Designing PbSe nanowires and nanorings through oriented attachment of nanoparticles. J. Am. Chem. Soc. 127, 7140–7147 (2005).
Patla, I. et al. Synthesis, two-dimensional assembly, and surface pressure-induced coalescence of ultranarrow PbS nanowires. Nano Lett. 7, 1459–1462 (2007).
Kim, H. et al. Parallel patterning of nanoparticles via electrodynamic focusing of charged aerosols. Nature Nanotech. 1, 117–121 (2006).
Ryan, K. M., Mastroianni, A., Stancil, K. A., Liu, H. T. & Alivisatos, A. P. Electric-field-assisted assembly of perpendicularly oriented nanorod superlattices. Nano Lett. 6, 1479–1482 (2006).
Joselevich, E. & Lieber, C. M. Vectorial growth of metallic and semiconducting single-wall carbon nanotubes. Nano Lett. 2, 1137–1141 (2002).
Sukhorukov, G. B., Antipov, A. A., Voigt, A., Donath, E. & Möhwald, H. pH-controlled macromolecule encapsulation in and release from polyelectrolyte multilayer nanocapsules. Macromol. Rapid Comm. 22, 44–46 (2001).
Kolny, J., Kornowski, A. & Weller, H. Self-organization of cadmium sulfide and gold nanoparticles by electrostatic interaction. Nano Lett. 2, 361–364 (2002).
Simard, J., Briggs, C., Boal, A. K. & Rotello, V. M. Formation and pH-controlled assembly of amphiphilic gold nanoparticles. Chem. Comm. 1943–1944 (2000).
Zhang, H., Zhou, Z., Yang, B. & Gao, M. Y. The influence of carboxyl groups on the photoluminescence of mercaptocarboxylic acid-stabilized CdTe nanoparticles. J. Phys. Chem. B 107, 8–13 (2003).
Tripp, S. L., Pusztay, S. V., Ribbe, A. E. & Wei, A. Self-assembly of cobalt nanoparticle rings. J. Am. Chem. Soc. 124, 7914–7915 (2002).
Terheiden, A., Dmitrieva, O., Acet, M. & Mayer, C. Magnetic field induced self-assembly of gas phase prepared FePt nanoparticles. Chem. Phys. Lett. 431, 113–117 (2006).
Morkved, T. L. et al. Local control of microdomain orientation in diblock copolymer thin films with electric fields. Science 273, 931–933 (1996).
Velev, O. D. & Bhatt, K. H. On-chip micromanipulation and assembly of colloidal particles by electric fields. Soft Matter 2, 738–750 (2006).
Benz, M., Rosenberg, K. J., Kramer, E. J. & Israelachvili, J. N. The deformation and adhesion of randomly rough and patterned surfaces. J. Phys. Chem. B 110, 11884–11893 (2006).
Huang, Y., Duan, X. F., Wei, Q. Q. & Lieber, C. M. Directed assembly of one-dimensional nanostructures into functional networks. Science 291, 630–633 (2001).
Hyun, S., Pei, L., Molinari, J. F. & Robbins, M. O. Finite-element analysis of contact between elastic self-affine surfaces. Phys. Rev. E 70, 026117 (2004).
Israelachvili, J. & Gourdon, D. Putting liquids under molecular-scale confinement. Science 292, 867–868 (2001).
Gerstenberg, M. C., Pedersen, J. S. & Smith, G. S. Surface induced ordering of micelles at the solid-liquid interface. Phys. Rev. E 58, 8028–8031 (1998).
Kocevar, K. & Musevic, I. Structural forces near phase transitions of liquid crystals. Chem. Phys. Chem. 4, 1049–1056 (2003).
Akbulut, M. et al. Forces between surfaces across nanoparticle solutions: Role of size, shape, and concentration. Langmuir 23, 3961–3969 (2007).
Min, Y. et al. Normal and shear forces generated during the ordering (directed assembly) of confined straight and curved nanowires. Nano Lett. 8, 246–252 (2008).
Lazarov, G. S., Denkov, N. D., Velev, O. D., Kralchevsky, P. A. & Nagayama, K. Formation of 2-dimensional structures from colloidal particles on fluorinated oil substrate. J. Chem. Soc.-Faraday Trans. 90, 2077–2083 (1994).
Nikoobakht, B., Wang, Z. L. & El-Sayed, M. A. Self-assembly of gold nanorods. J. Phys. Chem. B 104, 8635–8640 (2000).
Ahmed, S. & Ryan, K. M. Self-assembly of vertically aligned nanorod supercrystals using highly oriented pyrolytic graphite. Nano Lett. 7, 2480–2485 (2007).
Alig, A. R. G., Akbulut, M., Golan, Y. & Israelachvili, J. Forces between surfactant-coated ZnS nanoparticles in dodecane: Effect of water. Adv. Funct. Mater. 16, 2127–2134 (2006).
Prevo, B. G., Kuncicky, D. M. & Velev, O. D. Engineered deposition of coatings from nano- and micro-particles: A brief review of convective assembly at high volume fraction. Colloids Surf. A 311, 2–10 (2007).
Carpick, R. W., Agrait, N., Ogletree, D. F. & Salmeron, M. Variation of the interfacial shear strength and adhesion of a nanometer-sized contact. Langmuir 12, 3334–3340 (1996).
Ruths, M. Friction of mixed and single-component aromatic monolayers in contacts of different adhesive strength. J. Phys. Chem. B 110, 2209–2218 (2006).
Landman, U., Luedtke, W. D., Burnham, N. A. & Colton, R. J. Atomistic mechanisms and dynamics of adhesion, nanoindentation, and fracture. Science 248, 454–461 (1990).
Golan, Y. et al. Microtribology and direct force measurement of WS2 nested fullerene-like nanostructures. Adv. Mater. 11, 934–937 (1999).
Akbulut, M., Belman, N., Golan, Y. & Israelachvili, J. Frictional properties of confined nanorods. Adv. Mater. 18, 2589–2592 (2006).
Narayanan, R. & El-Sayed, M. A. Catalysis with transition metal nanoparticles in colloidal solution: Nanoparticle shape dependence and stability. J. Phys. Chem. B 109, 12663–12676 (2005).
Schwuger, M. J., Stickdorn, K. & Schomacker, R. Microemulsions in technical processes. Chem. Rev. 95, 849–864 (1995).
Smith, R. D., Fulton, J. L., Blitz, J. P. & Tingey, J. M. Reverse micelles and microemulsions in near-critical and supercritical fluids. J. Phys. Chem. 94, 781–787 (1990).
Kroto, H. W., Heath, J. R., Obrien, S. C., Curl, R. F. & Smalley, R. E. C60: Buckminsterfullerene. Nature 318, 162–163 (1985).
Iijima, S. Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991).
Tenne, R., Margulis, L., Genut, M. & Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nature 360, 444–446 (1992).
Tenne, R. Inorganic nanotubes and fullerene-like nanoparticles. Nature Nanotech. 1, 103–111 (2006).
Murphy, C. J. et al. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 109, 13857–13870 (2005).
Cozzoli, P. D., Kornowski, A. & Weller, H. Low-temperature synthesis of soluble and processable organic-capped anatase TiO2 nanorods. J. Am. Chem. Soc. 125, 14539–14548 (2003).
Kim, Y. H., Jun, Y. W., Jun, B. H., Lee, S. M. & Cheon, J. W. Sterically induced shape and crystalline phase control of GaP nanocrystals. J. Am. Chem. Soc. 124, 13656–13657 2002).
Guzelian, A. A. et al. Synthesis of size-selected, surface-passivated InP nanocrystals. J. Phys. Chem. 100, 7212–7219 (1996).
Steiner, D. et al. Zero-dimensional and quasi one-dimensional effects in semiconductor nanorods. Nano Lett. 4, 1073–1077 (2004).
Peng, X. G. et al. Shape control of CdSe nanocrystals. Nature 404, 59–61 (2000).
Halpert, J. E., Porter, V. J., Zimmer, J. P. & Bawendi, M. G. Synthesis of CdSe/CdTe nanobarbells. J. Am. Chem. Soc. 128, 12590–12591 (2006).
Yu, W. W., Wang, Y. A. & Peng, X. G. Formation and stability of size-, shape-, and structure-controlled CdTe nanocrystals: Ligand effects on monomers and nanocrystals. Chem. Mater. 15, 4300–4308 (2003).
Acharya, S., Panda, A. B., Belman, N., Efrima, S. & Golan, Y. A semiconductor-nanowire assembly of ultrahigh junction density by the Langmuir-Blodgett technique. Adv. Mater. 18, 210–213 (2006).
Markovich, G. et al. Architectonic quantum dot solids. Acc. Chem. Res. 32, 415–423 (1999).
Yang, P. D. Wires on water. Nature 425, 243–244 (2003).
Pradhan, N. & Efrima, S. Single-precursor, one-pot versatile synthesis under near ambient conditions of tunable, single and dual band fluorescing metal sulfide nanoparticles. J. Am. Chem. Soc. 125, 2050–2051 (2003).
Panda, A. B., Acharya, S., Efrima, S. & Golan, Y. Synthesis, assembly, and optical properties of shape- and phase-controlled ZnSe nanostructures. Langmuir 23, 765–770 (2007).
Acharya, S., Panda, A. B., Efrima, S. & Golan, Y. Polarization properties and switchable assembly of ultranarrow ZnSe nanorods. Adv. Mater. 19, 1105–1108 (2007).
Acharya, S., Patla, I., Kost, J., Efrima, S. & Golan, Y. Switchable assembly of ultra narrow CdS nanowires and nanorods. J. Am. Chem. Soc. 128, 9294–9295 (2006).
Pradhan, N. & Efrima, S. Supercrystals of uniform nanorods and nanowires, and the nanorod-to-nanowire oriented transition. J. Phys. Chem. B 108, 11964–11970 (2004).
Acharya, S. & Efrima, S. Two-dimensional pressure-driven nanorod-to-nanowire reactions in Langmuir monolayers at room temperature. J. Am. Chem. Soc. 127, 3486–3490 (2005).
Tang, Z. Y., Kotov, N. A. & Giersig, M. Spontaneous organization of single CdTe nanoparticles into luminescent nanowires. Science 297, 237–240 (2002).
Zhang, Z. L., Tang, Z. Y., Kotov, N. A. & Glotzer, S. C. Simulations and analysis of self-assembly of CdTe nanoparticles into wires and sheets. Nano Lett. 7, 1670–1675 (2007).
Tang, Z. Y., Zhang, Z. L., Wang, Y., Glotzer, S. C. & Kotov, N. A. Self-assembly of CdTe nanocrystals into free-floating sheets. Science 314, 274–278 (2006).
Xia, Y. N., Gates, B. & Park, S. H. Fabrication of three-dimensional photonic crystals for use in the spectral region from ultraviolet to near-infrared. J. Lightwave Technol. 17, 1956–1962 (1999).
Kumacheva, E., Garstecki, P., Wu, H. K. & Whitesides, G. M. Two-dimensional colloid crystals obtained by coupling of flow and confinement. Phys. Rev. Lett. 91, 128301 (2003).
Subramanian, G., Manoharan, V. N., Thorne, J. D. & Pine, D. J. Ordered macroporous materials by colloidal assembly: A possible route to photonic bandgap materials. Adv. Mater. 11, 1261–1265 (1999).
Jana, N. R. Shape effect in nanoparticle self-assembly. Angew. Chem. Int. Ed. 43, 1536–1540 (2004).
Velamakanni, B. V., Chang, J. C., Lange, F. F. & Pearson, D. S. New method for efficient colloidal particle packing via modulation of repulsive lubricating hydration forces. Langmuir 6, 1323–1325 (1990).
Lange, F. F. Powder processing science and technology for increased reliability. J. Am. Ceram. Soc. 72, 3–15 (1989).
Bhushan, B. Nanotribology and Nanomechanics: An Introduction (Springer, Berlin, 2005).
Xu, L. B. et al. Synthesis and magnetic behavior of periodic nickel sphere arrays. Adv. Mater. 15, 1562–1564 (2003).
Cheng, J. Y., Mayes, A. M. & Ross, C. A. Nanostructure engineering by templated self-assembly of block copolymers. Nature Mater. 3, 823–828 (2004).
Gourdon, D. et al. Mechanical and structural properties of BaCrO4 nanorod films under confinement and shear. Adv. Funct. Mater. 14, 238–242 (2004).
Fraenkelconrat, H. & Singer, B. Reconstitution of tobacco mosaic virus. 3. Improved methods and the use of mixed nucleic acids. Biochim. Biophys Acta 33, 359–370 (1959).
Butler, P. J. G. The current picture of the structure and assembly of tobacco mosaic virus. J. Gen. Virol. 65, 253–279 (1984).
Wijaya, A. & Flamad-Schifferli, K. High-density encapsulation of Fe3O4 nanoparticles in lipid vesicles. Langmuir 23, 9546–9550 (2007).
Lian, T. & Ho, R. J. Y. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 90, 667–680 (2001).
Wang, H. F. et al. In vitro and in vivo two-photon luminescence imaging of single gold nanorods. Proc. Natl Acad. Sci. USA 102, 15752–15756 (2005).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Landis, W. J. The strength of a calcified tissue depends in part on the molecular-structure and organization of its constituent mineral crystals in their organic matrix. Bone 16, 533–544 (1995).
Gwynn, I., Wade, S., Ito, K. & Richards, R. G. Novel aspects to the structure of rabbit articular cartilage. European Cells Mater. 4, 18–29 (2002).
Lee, H., Lee, B. P. & Messersmith, P. B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448, 338–341 (2007).
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
You, C. C., Verma, A. & Rotello, V. M. Engineering the nanoparticle-biomacromolecule interface. Soft Matter 2, 190–204 (2006).
Yang, Z. Q., Huck, W. T. S., Clarke, S. M., Tajbakhsh, A. R. & Terentjev, E. M. Shape-memory nanoparticles from inherently non-spherical polymer colloids. Nature Mater. 4, 486–490 (2005).
Sonnichsen, C. et al. Drastic reduction of plasmon damping in gold nanorods. Phys. Rev. Lett. 88, 077402 (2002).
Pelton, M. et al. Ultrafast resonant optical scattering from single gold nanorods: Large nonlinearities and plasmon saturation. Phys. Rev. B 73, 155419 (2006).
Wang, Z. L. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 104, 1153–1175 (2000).
Wiley, B. J., Xiong, Y. J., Li, Z. Y., Yin, Y. D. & Xia, Y. A. Right bipyramids of silver: A new shape derived from single twinned seeds. Nano Lett. 6, 765–768 (2006).
Raviv, U. et al. Cationic liposome-microtubule complexes: Pathways to the formation of two-state lipid-protein nanotubes with open or closed ends. Proc. Natl Acad. Sci. USA 102, 11167–11172 (2005).
Schneider, G. & Decher, G. From functional core/shell nanoparticles prepared via layer-by-layer deposition to empty nanospheres. Nano Lett. 4, 1833–1839 (2004).
Teranishi, T., Inoue, Y., Nakaya, M., Oumi, Y. & Sano, T. Nanoacorns: Anisotropically phase-segregated CoPd sulfide nanoparticles. J. Am. Chem. Soc. 126, 9914–9915 (2004).
Yoshimura, S., Takano, K. & Hachisu, S. in Polymer Colloids II (ed. Fitch, R. M.) 139 (Plenum, New York, 1980).
Manna, L., Scher, E. C. & Alivisatos, A. P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 122, 12700–12706 (2000).
Goddard, T. D., Huang, C. C. & Ferrin, T. E. Software extensions to UCSF Chimera for interactive visualization of large molecular assemblies. Structure 13, 473–482 (2005).
Yoo, P. J. et al. Spontaneous assembly of viruses on multilayered polymer surfaces. Nature Mater. 5, 234–240 (2006).
Ewert, K. K. et al. A columnar phase of dendritic lipid-based cationic liposome-DNA complexes for gene delivery: Hexagonally ordered cylindrical micelles embedded in a DNA honeycomb lattice. J. Am. Chem. Soc. 128, 3998–4006 (2006).
Autumn, K. et al. Adhesive force of a single gecko foot-hair. Nature 405, 681–685 (2000).
Acknowledgements
Y.G. and J.I. thank the US-Israel Binational Science Foundation for Grant #2006032; MA was supported by ONR award no. N00014-05-1-0540 and by BASF Corporation-002058; the sections on friction (J.I. and Y.M.) were supported by DOE Grant DE-FG02-87ER45331, and the work on carbon nanotubes (K.K.) was supported by Norwegian Research Council grant no. 166731. This work was also partially funded by the MRSEC Program of the NSF under award number DMR05-20415. We thank Wren Greene and Noshir Pesika for their helpful comments on the ms.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, Y., Akbulut, M., Kristiansen, K. et al. The role of interparticle and external forces in nanoparticle assembly. Nature Mater 7, 527–538 (2008). https://doi.org/10.1038/nmat2206
Issue Date:
DOI: https://doi.org/10.1038/nmat2206
This article is cited by
-
Photochromism from wavelength-selective colloidal phase segregation
Nature (2023)
-
Electrochemical grippers based on the tuning of surface forces for applications in micro- and nanorobotics
Scientific Reports (2023)
-
Self-assembled photonic cavities with atomic-scale confinement
Nature (2023)
-
Visualizing interfacial collective reaction behaviour of Li–S batteries
Nature (2023)
-
Statistical Optimization and Desirability Function for Producing Nano Silica from Dune Sand by Sol–gel Method Towards Methylene Blue Dye Removal
Journal of Inorganic and Organometallic Polymers and Materials (2023)