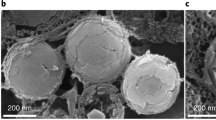
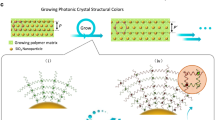
Abstract
Reflectins, a recently identified protein family that is enriched in aromatic and sulphur-containing amino acids, are used by certain cephalopods to manage and manipulate incident light in their environment. These proteins are the predominant constituent of nanoscaled photonic structures that function in static and adaptive colouration, extending visual performance and intra-species communication. Our investigation into recombinantly expressed reflectin has revealed unanticipated self-assembling and behavioural properties, and we demonstrate that reflectin can be easily processed into thin films, photonic grating structures and fibres. Our findings represent a key step in our understanding of the property–function relationships of this unique family of reflective proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vukusic, P. & Sambles, J. R. Photonic structures in biology. Nature 424, 852–855 (2003).
Onslow, H. On a periodic structure in many insect scales and the cause of their iridescent colours. Phil. Trans. R. Soc. Lond. B 211, 1–74 (1921).
Berthier, S. Iridescences: The Physical Colours of Insects (Springer, Berlin, 2006).
Johnsen, S. Cryptic and conspicuous colouration in the pelagic environment. Proc. R. Soc. Lond. B 269, 243–256 (2002).
Johnsen, S. & Sosik, H. M. Cryptic colouration and mirror sides as camouflage strategies in near-surface pelagic habitats: Implications for foraging and predator avoidance. Limnol. Oceanogr. 48, 1277–1288 (2003).
Griffiths, D. J., Winsor, H. & Luong-Van, T. Iridophores in the mantle of giant clams. Aust. Zool. 40, 319–326 (1992).
Crookes, W. J. et al. Reflectins: The unusual proteins of squid reflective tissues. Science 303, 235–238 (2004).
Land, M. F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 24, 75–106 (1972).
Denton, E. J., Land, F. R. S. & Land, M. F. Mechanisms of reflexion in silvery layers of fish and cephalopods. Proc. R. Soc. Lond. A 178, 43–61 (1971).
Denton, E. J. On the organization of reflecting surfaces in some marine animals. Phil. Trans. R. Soc. B 258, 285–313 (1970).
McFall-Ngai, M. J. & Montgomery, M. K. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae). Biol. Bull. 147, 332–339 (1990).
Montgomery, M. K. & McFall-Ngai, M. J. The muscle-derived lens of a squid bioluminescent organ is biochemically convergent with the ocular lens. Evidence for recruitment of aldehyde dehydrogenase as a predominant structural protein. J. Biol. Chem. 267, 20999–21003 (1992).
Weiss, J. L. et al. Methionine-rich repeat proteins: A family of membrane-associated proteins which contain unusual repeat regions. Biochim. Biophys. Acta 1668, 164–174 (2005).
Cooper, K. M. & Hanlon, R. T. Correlation of iridescence with changes in iridophore platelet ultrastructure in the squid Lolliguncula brevis. J. Exp. Biol. 121, 451–455 (1986).
Cooper, K. M., Hanlon, R. T. & Budelmann, B. U. Physiological colour change in squid iridophores II. Ultrastructural mechanisms in Lolliguncula brevis. Cell Tissue Res. 259, 15–24 (1990).
Mäthger, L. M., Collins, T. F. T. & Lima, P. A. The role of muscarinic receptors and intracellular Ca2+ in the spectral reflectivity changes of squid iridophores. J. Exp. Biol. 207, 1759–1769 (2004).
Fincham, A. G., Moradian-Oldak, J. & Simmer, J. P. The structural biology of developing dental enamel. J. Struct. Biol. 126, 270–299 (1999).
Eastoe, J. E. Organic matrix of tooth enamel. Nature 187, 411–412 (1960).
Du, C., Falini, G., Fermani, S., Abbot, C. & Moradian-Oldak, J. Supramolecular assembly of amelogenin nanospheres into birefringent microribbons. Science 307, 1450–1454 (2005).
Makin, O. S. & Serpell, L. C. Structures for amyloid fibrils. Fed. Eur. Bioch. Soc. J. 272, 5950–5961 (2005).
Swatloski, R. P., Spear, R. K., Holbrey, J. D. & Rogers, R. D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 124, 4974–4975 (2002).
Phillips, D. M. et al. Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids. J. Am. Chem. Soc. 126, 14350–14351 (2004).
Sundd, M., Kundu, S. & Jagannadham, M. V. Alcohol-induced conformational transitions in ervatamin C. An alpha-helix to beta-sheet switchover. J. Protein Chem. 19, 169–176 (2000).
Kumaran, S. & Roy, R. P. Helix-enhancing propersity of fluoro and alkyl alcohols: Influence of pH, temperature and cosolvent concentration on the helical conformation of peptides. J. Pep. Res. 53, 284–293 (1999).
Ober, C. Persistence pays off. Science 296, 859–861 (2002).
Qi, M. et al. A three-dimensional optic photonic crystal with designed point defects. Nature 429, 538–542 (2004).
Acknowledgements
This work has been financially supported by DARPA (DSO) and AFOSR. We acknowledge M. McFall-Ngai (NIH AI50611) and E.G. Ruby (NIH RR 12294) for providing us with squid tissue. We thank M. Gupta for assistance with white-light interferometry imaging and D. Morse, R. Hanlon and M. Yustak for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1-S6 (PDF 894 kb)
Rights and permissions
About this article
Cite this article
Kramer, R., Crookes-Goodson, W. & Naik, R. The self-organizing properties of squid reflectin protein. Nature Mater 6, 533–538 (2007). https://doi.org/10.1038/nmat1930
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1930
This article is cited by
-
Design and fabrication of recombinant reflectin-based multilayer reflectors: bio-design engineering and photoisomerism induced wavelength modulation
Scientific Reports (2021)
-
Cephalopod-inspired optical engineering of human cells
Nature Communications (2020)
-
How to define and study structural proteins as biopolymer materials
Polymer Journal (2020)
-
Dynamic pigmentary and structural coloration within cephalopod chromatophore organs
Nature Communications (2019)
-
Reconstruction of Dynamic and Reversible Color Change using Reflectin Protein
Scientific Reports (2019)