Abstract
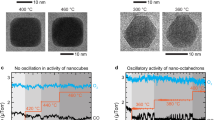
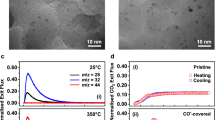
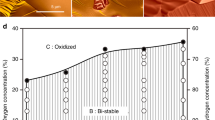
Many catalytic reactions under fixed conditions exhibit oscillatory behaviour. The oscillations are often attributed to dynamic changes in the catalyst surface. So far, however, such relationships were difficult to determine for catalysts consisting of supported nanoparticles. Here, we employ a nanoreactor to study the oscillatory CO oxidation catalysed by Pt nanoparticles using time-resolved high-resolution transmission electron microscopy, mass spectrometry and calorimetry. The observations reveal that periodic changes in the CO oxidation are synchronous with a periodic refacetting of the Pt nanoparticles. The oscillatory reaction is modelled using density functional theory and mass transport calculations, considering the CO adsorption energy and the oxidation rate as site-dependent. We find that to successfully explain the oscillations, the model must contain the phenomenon of refacetting. The nanoreactor approach can thus provide atomic-scale information that is specific to surface sites. This will improve the understanding of dynamic properties in catalysis and related fields.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nature Chem. 1, 37–46 (2009).
Newton, M. A., Belver-Coldeira, C., Martínez-Arias, A. & Fernandez-García, M. Dynamic in situ observation of rapid size and shape change of supported Pd nanoparticles during CO/NO cycling. Nature Mater. 6, 528–532 (2007).
Tao, F. et al. Reaction-driven restructuring of Rh–Pd and Pt–Pd core-shell nanoparticles. Science 322, 932–934 (2008).
Yoshida, H. et al. Temperature-dependent change in shape of platinum nanoparticles supported on CeO2 during catalytic reactions. Appl. Phys. Exp. 4, 065001 (2011).
Hansen, P. L. et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 295, 2053–2055 (2002).
Boyes, E. D. & Gai, P. L. Environmental high resolution electron microscopy and applications to chemical science. Ultramicroscopy 67, 219–232 (1997).
Thomas, J. M. & Somorjai, G. A. (eds) Top. Catal. 8 (special issue), 1–140 (1999).
Campbell, C. T. Catalysts under pressure. Science 294, 1471–1472 (2001).
Buurmans, I. L. C. & Weckhuysen, B. M. Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy. Nature Chem. 4, 873–886 (2012).
Topsøe, H. Developments in operando studies and in situ characterization of heterogeneous catalysts. J. Catalys. 216, 155–164 (2003).
Freund, H. J., Meijer, G., Scheffler, M., Schlögl, R. & Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 50, 10064–10094 (2011).
Imbihl, R. & Ertl, G. Oscillatory kinetics in heterogeneous catalysis. Chem. Rev. 95, 697–733 (1995).
Liauw, M. A., Plath, P. J. & Jaeger, N. I. Complex oscillations and global coupling during the catalytic oxidation of CO. J. Chem. Phys. 104, 6375–6386 (1996).
Matera, S. & Reuter, K. Transport limitations and bistability for in situ CO oxidation at RuO2 (110): First-principles based multiscale modeling. Phys. Rev. B 82, 085446 (2010).
Turner, J. E., Sales, B. C. & Maple, M. B. Oscillatory oxidation of CO over a Pt catalyst. Surf. Sci. 103, 54–74 (1981).
Hendriksen, B. L. M., Bobaru, S. C. & Frenken, J. W. M. Bistability and oscillations in CO oxidation studied with scanning tunneling microscopy inside a reactor. Catal. Today 105, 234–243 (2005).
Harris, P. J. F. Sulphur-induced faceting of platinum catalyst particles. Nature 323, 792–794 (1986).
Contard, L. C. et al. Aberration-corrected imaging of active sites on industrial catalyst nanoparticles. Angew. Chem. Int. Ed. 46, 3683–3685 (2007).
Hansen, L. P. et al. Atomic-scale edge structures on industrial-style MoS2 nanocatalysts. Angew. Chem. Int. Ed. 50, 10153–10156 (2011).
Giorgio, S. et al. Environmental electron microscopy (ETEM) for catalysts with a closed e-cell with carbon windows. Ultramicroscopy 106, 503–507 (2006).
Creemer, J. F. et al. Atomic-scale electron microscopy at ambient pressure. Ultramicroscopy 337, 209–212 (2008).
Creemer, J. F. et al. Proc. 2011 IEEE 24th Int. Conf. MEMS 1103–1106 (IEEE, 2011).
Allard, L. F. et al. Novel MEMS-based gas-cell/heating specimen holder provides advanced imaging capabilities for in situ reaction studies. Microsc. Microanal. 18, 656–666 (2012).
Baker, R. T. K. In situ electron microscopy studies of catalyst particle behavior. Catal. Rev. Sci. Eng. 19, 161–209 (1979).
Sharma, R. & Crozier, P. in Handbook of Microscopy for Nanotechnology Vol. 531 (eds Yao, N. & Wang, Z. L.) (Kluwer Academic Publishers, 2005).
Chenna, S. & Crozier, P. A. Operando transmission electron microscopy: A technique for detection of catalysis using electron energy-loss spectroscopy in the transmission electron microscope. ACS Catal. 2, 2395–2402 (2012).
Williamson, M. J., Tromp, R. M., Vereecken, P. M., Hull, R. & Ross, F. M. Dynamic microscopy of nanoscale cluster growth at the solid–liquid interface. Nature Mater. 2, 532–536 (2003).
Jensen, R. et al. Self-sustained carbon monoxide oxidation oscillations on size-selected platinum nanoparticles at atmospheric pressure. Phys. Chem. Chem. Phys. 15, 2698–2702 (2013).
Ackermann, M. D. et al. Structure and reactivity of surface oxides on Pt(110) during catalytic CO oxidation. Phys. Rev. Lett. 95, 255505 (2005).
Li, W. X. et al. Oxidation of Pt(110). Phys. Rev. Lett. 30, 146104 (2004).
Jiang, T. et al. Trends in CO oxidation rates for metal nanoparticles and close-packed, stepped, and kinked surfaces. J. Phys. Chem. C 113, 10548–10553 (2009).
Falsig, H. et al. On the structure sensitivity of direct NO decomposition over low-index transition metals facets. Top. Catal. 57, 80–88 (2014).
Carlsson, P. A., Zhdanov, V. P. & Skoglundh, M. Self-sustained kinetic oscillations in CO oxidation over silica-supported Pt. Phys. Chem. Chem. Phys. 8, 2703–2706 (2006).
Thostrup, P. et al. Adsorption-induced step formation. Phys. Rev. Lett. 87, 126102 (2001).
Tao, F. et al. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science 327, 850–853 (2010).
Vogel, D. et al. Local catalytic ignition during CO oxidation on low-index Pt and Pd surfaces: A combined PEEM, MS, and DFT study. Angew. Chem. Int. Ed. 51, 10041–10044 (2012).
Johánek, V. et al. Fluctuations and bistabilities on catalyst nanoparticles. Science 304, 1639–1644 (2004).
Gorodetskii, V. et al. Coupling between adjacent crystal planes in heterogeneous catalysis by propagating reaction–diffusion waves. Nature 370, 276–279 (1994).
Robertson, J. K. & Wise, K. D. Modeling a microfluidic system using Knudsen’s empirical equation for flow in the transition regime. J. Vac. Sci. Technol. A 19, 358–364 (2001).
Vendelbo, S. B. et al. Method for local temperature measurement in a nanoreactor for in situ high-resolution electron microscopy. Ultramicroscopy 133, 72–79 (2013).
Jinchek, J. R. & Helveg, S. Image resolution and sensitivity in an environmental transmission electron microscope. Micron 11, 1156–1168 (2012).
Acknowledgements
This work was performed in the framework of NIMIC (Nano IMaging under Industrial Conditions), a SmartMix project of the Dutch Ministry of Economic Affairs. The authors acknowledge support from J.C. Wolff and J. van Wingerden (DIMES Technology Centre), G.J.C. van Baarle (Leiden Probe Microscopy BV), and M. Thorhauge and S. Ullmann (Haldor Topsøe A/S). The authors acknowledge Ib Chorkendorff (Technical University of Denmark) for fruitful discussions. Haldor Topsøe A/S is acknowledged for access to its electron microscopy facility. The work is dedicated to the legacy of H. Topsøe and his 100 years of dedication to catalysis and fundamental science.
Author information
Authors and Affiliations
Contributions
J.F.C., B.M. and L.M. developed and produced the nanoreactors. S.B.V. and P.D. designed and built the specimen holder. S.B.V. and I.P. prepared samples. S.B.V., C.F.E., P.J.K. and S.H. performed the experiments. S.B.V., C.F.E. and S.H. analysed the data. H.F. performed microkinetic modelling. S.B.V. performed reactor simulations. S.H., S.B.V., C.F.E. and H.F. composed the manuscript and it was critically discussed and revised together with J.F.C., P.J.K., B.J.N. and R.R. The project was supervised by S.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3438 kb)
Supplementary Movie 1
Supplementary Movie 1 (MOV 15030 kb)
Supplementary Movie 2
Supplementary Movie 2 (MOV 12194 kb)
Rights and permissions
About this article
Cite this article
Vendelbo, S., Elkjær, C., Falsig, H. et al. Visualization of oscillatory behaviour of Pt nanoparticles catalysing CO oxidation. Nature Mater 13, 884–890 (2014). https://doi.org/10.1038/nmat4033
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4033
This article is cited by
-
Time-resolved transmission electron microscopy for nanoscale chemical dynamics
Nature Reviews Chemistry (2023)
-
Selective Hydrogenation of Croton Aldehyde on Pt Nanoparticles Controlled by Tailoring Fraction of Well-Ordered Facets Under Different Pretreatment Conditions
Catalysis Letters (2023)
-
Periodic structural changes in Pd nanoparticles during oscillatory CO oxidation reaction
Nature Communications (2022)
-
Nanokelvin-resolution thermometry with a photonic microscale sensor at room temperature
Nature Photonics (2022)
-
The concept of active site in heterogeneous catalysis
Nature Reviews Chemistry (2022)