Abstract
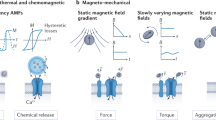
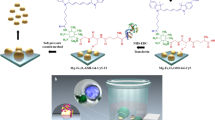
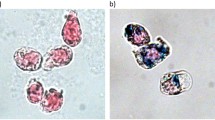
The regulation of cellular activities in a controlled manner is one of the most challenging issues in fields ranging from cell biology to biomedicine1,2. Nanoparticles have the potential of becoming useful tools for controlling cell signalling pathways in a space and time selective fashion3,4. Here, we have developed magnetic nanoparticles that turn on apoptosis cell signalling by using a magnetic field in a remote and non-invasive manner. The magnetic switch consists of zinc-doped iron oxide magnetic nanoparticles5 (Zn0.4Fe2.6O4), conjugated with a targeting antibody for death receptor 4 (DR4) of DLD-1 colon cancer cells. The magnetic switch, in its On mode when a magnetic field is applied to aggregate magnetic nanoparticle-bound DR4s, promotes apoptosis signalling pathways. We have also demonstrated that the magnetic switch is operable at the micrometre scale and that it can be applied in an in vivo system where apoptotic morphological changes of zebrafish are successfully induced.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hoffman, B. D., Grashoff, C. & Schwartz, M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Gorostiza, P. & Isacoff, E. Y. Optical switches for remote and noninvasive control of cell signaling. Science 322, 395–399 (2008).
Lee, S. E., Liu, G. L., Kim, F. & Lee, L. P. Remote optical switch for localized and selective control of gene interference. Nano Lett. 9, 562–570 (2009).
Chung, I. et al. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature 464, 783–787 (2010).
Jang, J-t. et al. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew. Chem. Int. Ed. 48, 1234–1238 (2009).
Jordan, J. D., Landau, E. M. & Iyengar, R. Signaling networks: The origins of cellular multitasking. Cell 103, 193–200 (2000).
Pankhurst, Q. A., Connolly, J., Jones, S. K. & Dobson, J. Application of magnetic nanoparticles in biomedicine. J. Phys. D 36, R167–R181 (2003).
Gupta, A. K. & Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4021 (2005).
Huang, H., Delikanli, S., Zeng, H., Ferkey, D. M. & Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nature Nanotech. 5, 602–606 (2010).
Creixell, M. et al. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 5, 7124–7129 (2011).
Wang, N., Butler, J. P. & Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993).
Kanczler, J. M. et al. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Eng. 16, 3241–3250 (2010).
Hughes, S., Haj, A. J. E. & Dobson, J. Magnetic micro- and nanoparticle mediated activation of mechanosensitive ion channels. Med. Eng. Phys. 27, 754–762 (2005).
Dobson, J. Remote control of cellular behavior with magnetic nanoparticles. Nature Nanotech. 3, 139–143 (2008).
Mannix, R. J. et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nature Nanotech. 3, 36–40 (2008).
Lee, J-H. et al. Artificial control of cell signaling and growth by magnetic nanoparticles. Angew. Chem. Int. Ed. 49, 5698–5702 (2010).
Danial, N. N. & Korsmeyer, S. J. Cell death: Critical control points. Cell 116, 205–219 (2004).
Williams, G. T. Programmed cell death: Apoptosis and oncogenesis. Cell 65, 1097–1098 (1991).
Storey, S. Targeting apoptosis: Selected anticancer strategies. Nature Rev. 7, 971–972 (2008).
Fulda, S. & Debatin, K-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25, 4798–4811 (2006).
Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nature Rev. Cancer 2, 420–430 (2002).
Sayers, T. J. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol. Immunother. 60, 1173–1180 (2011).
Mahalingam, D., Szegezdi, E., Keane, M., Jong, S. de & Samali, A. TRAIL receptor signaling and modulation: Are we on the right TRAIL? Cancer Treat. Rev. 35, 280–288 (2009).
Vondálová Blanárová, O. et al. Cisplatin and a potent platinum(IV) complex-mediated enhancement of TRAIL-induced cancer cells killing is associated with modulation of upstream events in the extrinsic apoptotic pathway. Carcinogenesis 32, 42–51 (2011).
Schneider-Brachert, W. et al. Compartmentalization of TNF receptor 1 signaling: Internalized TNF receptosomes as death signaling vesicles. Immunity 21, 415–428 (2004).
Kelley, S. K. & Ashkenazi, A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr. Opin. Pharmacol. 4, 333–339 (2004).
Ashkenazi, A. & Herbst, R. S. To kill a tumor cell: The potential of proapoptotic receptor agonists. J. Clin. Invest. 118, 1979–1990 (2008).
Meeker, D. C. Finite element method magnetics. http://www.femm.info (accessed Feb 9, 2011).
Türkcan, S. et al. Observing the confinement potential of bacterial pore-forming toxin receptors inside rafts with nonblinking Eu3+-doped oxide nanoparticles. Biophys. J. 102, 2299–2308 (2012).
Pralle, A., Keller, P., Florin, E-L., Simons, K. & Hörber, J. K. H. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1007 (2000).
Porter, A. G. & Jänicke, R. U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6, 99–104 (1999).
Pyati, U. J., Look, A. T. & Hammershmidt, M. Zebrafish as a powerful vertebrate model system for in vivo studies of cell death. Semin. Cancer Biol. 17, 154–165 (2007).
Eimon, P. M. & Ashkenazi, A. The zebrafish as a model organism for the study of apoptosis. Apoptosis 15, 331–349 (2010).
Yamashita, M. Apoptosis in zebrafish development. Comp. Biochem. Phys. B 136, 731–742 (2003).
Eimon, P. M. et al. Delineation of the cell-extrinsic apoptosis pathway in the zebrafish. Cell Death Differ. 13, 1619–1630 (2006).
Bobe, J. & Goetz, F. W. Molecular cloning and expression of a TNF receptor and two TNF ligands in the fish ovary. Comp. Biochem. Phys. B 129, 475–481 (2001).
Matsuda, H. The current trends and future prospects of regenerative medicine in cardiovascular diseases. Asian Cardiovasc. Thorac. Ann. 13, 101–102 (2005).
Oblak, A. & Jerala, R. Toll-like receptor 4 activation in cancer progression and therapy. Clin. Dev. Immunol. 475, 1–12 (2011).
Acknowledgements
This work was financially supported by grants from the Creative Research Initiative (2010-0018286), WCU Program (R32-10217), National Research Foundation of Korea (2011-0017611) and the second stage BK21 for Chemistry and Medical Sciences of Yonsei University. S.W.P. was supported by the National Research Foundation, Mid-career Researcher Program (72011-0043). M.H.C. was supported by a Hi Seoul Science/Humanities Fellowship from the Seoul Scholarship Foundation.
Author information
Authors and Affiliations
Contributions
J.C. and J-S.S. conceived and designed the experiments. M.H.C., E.J.L., M.S. and J-w.K. performed the experiments. S.W.P. provided advice on the in vivo zebrafish experiments. M.H.C., E.J.L., J-H.L., D.Y., J-S.S. and J.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2910 kb)
Rights and permissions
About this article
Cite this article
Cho, M., Lee, E., Son, M. et al. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nature Mater 11, 1038–1043 (2012). https://doi.org/10.1038/nmat3430
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3430
This article is cited by
-
Modulating cell signalling in vivo with magnetic nanotransducers
Nature Reviews Methods Primers (2022)
-
Progress in DNA Aptamers as Recognition Components for Protein Functional Regulation
Chemical Research in Chinese Universities (2022)
-
Photothermogenetic inhibition of cancer stemness by near-infrared-light-activatable nanocomplexes
Nature Communications (2020)
-
Assembled wearable mechanical sensor prepared based on cotton fabric
Journal of Materials Science (2020)
-
Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis
Nature Communications (2018)