Abstract
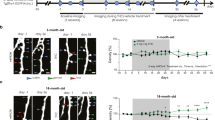
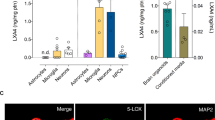
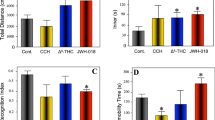
The balance between detrimental, pro-aging, often stochastic processes and counteracting homeostatic mechanisms largely determines the progression of aging. There is substantial evidence suggesting that the endocannabinoid system (ECS) is part of the latter system because it modulates the physiological processes underlying aging1,2. The activity of the ECS declines during aging, as CB1 receptor expression and coupling to G proteins are reduced in the brain tissues of older animals3,4,5 and the levels of the major endocannabinoid 2-arachidonoylglycerol (2-AG) are lower6. However, a direct link between endocannabinoid tone and aging symptoms has not been demonstrated. Here we show that a low dose of Δ9-tetrahydrocannabinol (THC) reversed the age-related decline in cognitive performance of mice aged 12 and 18 months. This behavioral effect was accompanied by enhanced expression of synaptic marker proteins and increased hippocampal spine density. THC treatment restored hippocampal gene transcription patterns such that the expression profiles of THC-treated mice aged 12 months closely resembled those of THC-free animals aged 2 months. The transcriptional effects of THC were critically dependent on glutamatergic CB1 receptors and histone acetylation, as their inhibition blocked the beneficial effects of THC. Thus, restoration of CB1 signaling in old individuals could be an effective strategy to treat age-related cognitive impairments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Di Marzo, V., Stella, N. & Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 16, 30–42 (2015).
Bilkei-Gorzo, A. The endocannabinoid system in normal and pathological brain ageing. Phil. Trans. R. Soc. Lond. B 367, 3326–3341 (2012).
Wang, L., Liu, J., Harvey-White, J., Zimmer, A. & Kunos, G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. USA 100, 1393–1398 (2003).
Berrendero, F. et al. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. Biophys. Acta 1407, 205–214 (1998).
Romero, J. et al. Loss of cannabinoid receptor binding and messenger RNA levels and cannabinoid agonist–stimulated [35S]guanylyl-5′O-(thio)-triphosphate binding in the basal ganglia of aged rats. Neuroscience 84, 1075–1083 (1998).
Piyanova, A. et al. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech. Ageing Dev. 150, 55–64 (2015).
Han, J. et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050 (2012).
Puighermanal, E., Busquets-Garcia, A., Maldonado, R. & Ozaita, A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Phil. Trans. R. Soc. Lond. B 367, 3254–3263 (2012).
Varvel, S.A., Anum, E., Niyuhire, F., Wise, L.E. & Lichtman, A.H. Δ9-THC-induced cognitive deficits in mice are reversed by the GABAA antagonist bicuculline. Psychopharmacology (Berl.) 178, 317–327 (2005).
Head, E. et al. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol. Aging 30, 1125–1134 (2009).
Morrison, J.H. & Baxter, M.G. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 13, 240–250 (2012).
Duce, J.A. et al. Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia 56, 106–117 (2008).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Semba, R.D. et al. Plasma klotho and mortality risk in older community-dwelling adults. J. Gerontol. A Biol. Sci. Med. Sci. 66, 794–800 (2011).
Dubal, D.B. et al. Life extension factor klotho enhances cognition. Cell Rep. 7, 1065–1076 (2014).
Cuenco, K.T. et al. Association of TTR polymorphisms with hippocampal atrophy in Alzheimer disease families. Neurobiol. Aging 32, 249–256 (2011).
Li, X. & Buxbaum, J.N. Transthyretin and the brain re-visited: is neuronal synthesis of transthyretin protective in Alzheimer's disease? Mol. Neurodegener. 6, 79 (2011).
Tanaka, J. et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319, 1683–1687 (2008).
Neidl, R. et al. Late-life environmental enrichment induces acetylation events and nuclear factor κB–dependent regulations in the hippocampus of aged rats showing improved plasticity and learning. J. Neurosci. 36, 4351–4361 (2016).
Erickson, K.I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 108, 3017–3022 (2011).
Gemma, C. & Bickford, P.C. Interleukin-1β and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev. Neurosci. 18, 137–148 (2007).
Khodosevich, K. et al. Connective tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. Neuron 79, 1136–1151 (2013).
Robison, A.J. & Nestler, E.J. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. 12, 623–637 (2011).
Derkinderen, P. et al. Regulation of extracellular signal–regulated kinase by cannabinoids in hippocampus. J. Neurosci. 23, 2371–2382 (2003).
Valjent, E. et al. Δ9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur. J. Neurosci. 14, 342–352 (2001).
Impey, S., Obrietan, K. & Storm, D.R. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 23, 11–14 (1999).
Kandel, E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 5, 14 (2012).
Villeda, S.A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014).
Fusco, S. et al. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc. Natl. Acad. Sci. USA 109, 621–626 (2012).
Saura, C.A. & Valero, J. The role of CREB signaling in Alzheimer's disease and other cognitive disorders. Rev. Neurosci. 22, 153–169 (2011).
West, A.E. et al. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. USA 98, 11024–11031 (2001).
Das, C. et al. Binding of the histone chaperone ASF1 to the CBP bromodomain promotes histone acetylation. Proc. Natl. Acad. Sci. USA 111, E1072–E1081 (2014).
Vecsey, C.G. et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. 27, 6128–6140 (2007).
Shieh, P.B., Hu, S.C., Bobb, K., Timmusk, T. & Ghosh, A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20, 727–740 (1998).
Gao, H. et al. Long-term dietary α-linolenic acid supplement alleviates cognitive impairment correlate with activating hippocampal CREB signaling in natural aging rats. Mol. Neurobiol. 53, 4772–4786 (2016)
Gräff, J. & Tsai, L.H. Histone acetylation: molecular mnemonics on the chromatin. Nat. Rev. Neurosci. 14, 97–111 (2013).
Peleg, S. et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328, 753–756 (2010).
Bilkei-Gorzo, A. et al. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc. Natl. Acad. Sci. USA 102, 15670–15675 (2005).
Albayram, O. et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl. Acad. Sci. USA 108, 11256–11261 (2011).
Piyanova, A. et al. Loss of CB1 receptors leads to decreased cathepsin D levels and accelerated lipofuscin accumulation in the hippocampus. Mech. Ageing Dev. 134, 391–399 (2013).
Monory, K. et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466 (2006).
Fischer, A., Sananbenesi, F., Wang, X., Dobbin, M. & Tsai, L.H. Recovery of learning and memory is associated with chromatin remodelling. Nature 447, 178–182 (2007).
Day, J.J. & Sweatt, J.D. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology 37, 247–260 (2012).
Zimmer, A., Zimmer, A.M., Hohmann, A.G., Herkenham, M. & Bonner, T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 96, 5780–5785 (1999).
Lastres-Becker, I., Molina-Holgado, F., Ramos, J.A., Mechoulam, R. & Fernández-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol. Dis. 19, 96–107 (2005).
Varvel, S.A. et al. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl.) 186, 226–234 (2006).
Marchalant, Y., Brothers, H.M. & Wenk, G.L. Cannabinoid agonist WIN-55,212-2 partially restores neurogenesis in the aged rat brain. Mol. Psychiatry 14, 1068–1069 (2009).
Galve-Roperh, I. et al. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal–regulated kinase activation. Nat. Med. 6, 313–319 (2000).
Sánchez, I., Mahlke, C. & Yuan, J. Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 421, 373–379 (2003).
Barnes, C.A., Suster, M.S., Shen, J. & McNaughton, B.L. Multistability of cognitive maps in the hippocampus of old rats. Nature 388, 272–275 (1997).
Sanchez-Mejia, R.O. et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat. Neurosci. 11, 1311–1318 (2008).
Cannich, A. et al. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn. Mem. 11, 625–632 (2004).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008).
Massó, A. et al. Secreted and transmembrane αklotho isoforms have different spatio-temporal profiles in the brain during aging and Alzheimer's disease progression. PLoS One 10, e0143623 (2015).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Miró, X. et al. Haploinsufficiency of the murine Polycomb gene Suz12 results in diverse malformations of the brain and neural tube. Dis. Model. Mech. 2, 412–418 (2009).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft grants FOR926 (SP2 and CP2), BI-1227/5 and SFB645. J.L.S. and A.Z. are members of the DFG Cluster of Excellence ImmunoSensation.
Author information
Authors and Affiliations
Contributions
O.A., A.B.-G., J.L.S., M.D.-G., I.B. and A.Z. designed research; O.A., A.P., S.I., T.U., H.O., I.R., K.M., A.D. and A.B.-G. performed research; O.A., A.P., A.B.-G., T.U., J.L.S. and A.Z. analyzed data; and O.A., A.B.-G., J.L.S. and A.Z. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–10 and Supplementary Tables 2–4 (PDF 42541 kb)
Supplementary Table 1
Ten modules identified from WGCNA, related to Supplementary Figure 7. (XLSX 647 kb)
Supplementary Data
Full images of immunoblots (PDF 44932 kb)
Rights and permissions
About this article
Cite this article
Bilkei-Gorzo, A., Albayram, O., Draffehn, A. et al. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med 23, 782–787 (2017). https://doi.org/10.1038/nm.4311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4311
This article is cited by
-
Cannabis Use and Cognitive Functioning Across the Lifespan
Current Addiction Reports (2024)
-
NAMPT encapsulated by extracellular vesicles from young adipose-derived mesenchymal stem cells treated tendinopathy in a “One-Stone-Two-Birds” manner
Journal of Nanobiotechnology (2023)
-
Chronic low-dose Δ9-tetrahydrocannabinol (THC) treatment stabilizes dendritic spines in 18-month-old mice
Scientific Reports (2023)
-
A longitudinal study of cannabis use and risk for cognitive and functional decline among older adults with HIV
AIDS and Behavior (2023)
-
Chronic exposure to a synthetic cannabinoid alters cerebral brain metabolism and causes long-lasting behavioral deficits in adult mice
Journal of Neural Transmission (2023)