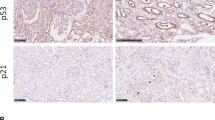
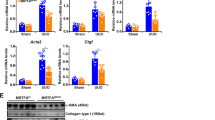
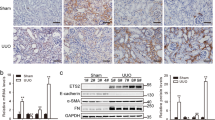
Abstract
Progressive kidney fibrosis contributes greatly to end-stage renal failure, and no specific treatment is available to preserve organ function. During renal fibrosis, myofibroblasts accumulate in the interstitium of the kidney, leading to massive deposition of extracellular matrix and organ dysfunction. The origin of myofibroblasts is manifold, but the contribution of an epithelial-to-mesenchymal transition (EMT) undergone by renal epithelial cells during kidney fibrosis is still debated. We show that the reactivation of Snai1 (encoding snail family zinc finger 1, known as Snail1) in mouse renal epithelial cells is required for the development of fibrosis in the kidney. Damage-mediated Snail1 reactivation induces a partial EMT in tubular epithelial cells that, without directly contributing to the myofibroblast population, relays signals to the interstitium to promote myofibroblast differentiation and fibrogenesis and to sustain inflammation. We also show that Snail1-induced fibrosis can be reversed in vivo and that obstructive nephropathy can be therapeutically ameliorated in mice by targeting Snail1 expression. These results reconcile conflicting data on the role of the EMT in renal fibrosis and provide avenues for the design of novel anti-fibrotic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
04 September 2015
In the version of this article initially published, the word 'genetically' was added by the editor to the penultimate sentence of the abstract during proofing of the manuscript. However, the authors used not only a genetic knockout approach but also morpholino-induced inhibition of proper mRNA processing to target Snail1 expression. Thus, the word 'genetically' has been deleted from the sentence to better convey the findings of the report. The error has been corrected in the HTML and PDF versions of the article.
References
Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684–696 (2011).
National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. No. 12–3895, http://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx#1 (2012).
Dressler, G. Tubulogenesis in the developing mammalian kidney. Trends Cell Biol. 12, 390–395 (2002).
Thiery, J.P., Acloque, H., Huang, R.Y. & Nieto, M.A. Epithelial–mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Boutet, A. et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 25, 5603–5613 (2006).
Sato, M., Muragaki, Y., Saika, S., Roberts, A.B. & Ooshima, A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 112, 1486–1494 (2003).
Lange-Sperandio, B. et al. Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am. J. Pathol. 171, 861–871 (2007).
Grande, M.T. et al. Deletion of H-Ras decreases renal fibrosis and myofibroblast activation following ureteral obstruction in mice. Kidney Int. 77, 509–518 (2010).
Chevalier, R.L., Forbes, M.S. & Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75, 1145–1152 (2009).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Humphreys, B.D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Li, L., Zepeda-Orozco, D., Black, R. & Lin, F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am. J. Pathol. 176, 1767–1778 (2010).
LeBleu, V.S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Rowe, R.G. et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol. 184, 399–408 (2009).
Shao, X., Somlo, S. & Igarashi, P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J. Am. Soc. Nephrol. 13, 1837–1846 (2002).
Franci, C. et al. Expression of Snail in tumor-stroma interface. Oncogene 25, 5134–5144 (2006).
de Oliveira Costa, M.Z., Bacchi, C.E. & Franco, M. Histogenesis of the acquired cystic kidney disease: an immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 14, 348–352 (2006).
Borges, F.T. et al. TGF-β-1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 24, 385–392 (2013).
Dhasarathy, A., Phadke, D., Mav, D., Shah, R.R. & Wade, P.A. The transcription factors Snail and Slug activate the transforming growth factor-β signaling pathway in breast cancer. PLoS ONE 6, e26514 (2011).
Grande, M.T., Pérez-Barriocanal, F. & López-Novoa, J.M. Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J. Inflamm. (Lond.) 7, 19 (2010).
Biswas, S.K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010).
Lyons, J.G. et al. Snail upregulates pro-inflammatory mediators and inhibits differentiation in oral keratinocytes. Cancer Res. 68, 4525–4530 (2008).
Hsu, D.S. et al. Acetylation of Snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell 26, 534–548 (2014).
Yang, H.-C., Zuo, Y. & Fogo, A.B. Models of chronic disease. Drug Discov. Today Dis. Models 7, 13–19 (2010).
Nieto, M.A. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342, 1234850 (2013).
Kriz, W., Kaissling, B. & Le Hir, M. Epithelial–mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 121, 468–474 (2011).
Fragiadaki, M. & Mason, R.M. Epithelial–mesenchymal transition in renal fibrosis - evidence for and against. Int. J. Exp. Pathol. 92, 143–150 (2011).
Zeisberg, M. & Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 21, 1819–1834 (2010).
Meng, X.M., Nikolic-Paterson, D.J. & Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 10, 493–503 (2014).
Vega, S. et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 18, 1131–1143 (2004).
Kim, N.H. et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J. Cell Biol. 195, 417–433 (2011).
Tian, X.J., Zhang, H. & Xing, J. Coupled reversible and irreversible bistable switches underlying TGFbeta-induced epithelial to mesenchymal transition. Biophys. J. 105, 1079–1089 (2013).
Morizane, R. et al. miR-34c attenuates epithelial-mesenchymal transition and kidney fibrosis with ureteral obstruction. Sci. Rep. 4, 4578 (2014).
Kalluri, R. & Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Liu, Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 69, 213–217 (2006).
López-Novoa, J.M. & Nieto, M.A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 1, 303–314 (2009).
Wu, Y. et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell 15, 416–428 (2009).
Rosenbloom, J., Castro, S.V. & Jimenez, S.A. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann. Intern. Med. 152, 159–166 (2010).
Zeisberg, M. et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 (2003).
Esteban, V. et al. Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J. Am. Soc. Nephrol. 15, 1514–1529 (2004).
Miyajima, A. et al. Novel nuclear factor kappa B activation inhibitor prevents inflammatory injury in unilateral ureteral obstruction. J. Urol. 169, 1559–1563 (2003).
Mehal, W.Z., Iredale, J. & Friedman, S.L. Scraping fibrosis: expressway to the core of fibrosis. Nat. Med. 17, 552–553 (2011).
Luedde, T. & Schwabe, R.F. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 8, 108–118 (2011).
Tsai, J.H., Donaher, J.L., Murphy, D.A., Chau, S. & Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736 (2012).
Ocaña, O.H. et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 (2012).
Rodríguez-López, A., Flores, O., Arévalo, M. & López-Novoa, J.M. Glomerular cell proliferation and apoptosis in the early phase of renal damage in uninephrectomized spontaneously hypertensive rats. Kidney Int. 54, S36–S40 (1998).
Acknowledgements
We thank members of A. Nieto's laboratory for their helpful discussions and comments throughout the years. We also thank S. Canals for his advice on statistical analysis, and C. Villena and S. Ingham for the design of Supplementary Figure 10. We are very grateful to P. Igarashi (University of Minnesota Medical School) for providing the Ksp1.3-Cre mice. This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (BFU2008-01042 and CONSOLIDER-INGENIO 2010 CSD2007-00023 and CDS2007-00017), the Generalitat Valenciana (Prometeo 2008/049 and PROMETEOII/2013/002) and the European Research Council (ERC AdG 322694) to M.A.N. The Instituto de Neurociencias is a Centre of Excellence Severo Ochoa. M.T.G. was a recipient of a contract from the Junta de Ampliación de Estudios program at the Consejo Superior de Investigaciones Científicas European Social Fund.
Author information
Authors and Affiliations
Contributions
M.T.G. and B.S.-L. performed the majority of experiments, analyzed the data and contributed to writing the manuscript. C.L.-B. was instrumental in the surgery, and histological and expression studies. C.A.F. and A.B. started the project, R.G.R. and S.J.W. provided the Snai1loxP mice before publication, J.M.L.-N. and M.A. quantified Sirius red staining and J.M.L.-N. helped in the interpretation of data. M.A.N. conceived the project, interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 & Supplementary Table 1 (PDF 13042 kb)
Rights and permissions
About this article
Cite this article
Grande, M., Sánchez-Laorden, B., López-Blau, C. et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21, 989–997 (2015). https://doi.org/10.1038/nm.3901
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3901
This article is cited by
-
WNT-dependent interaction between inflammatory fibroblasts and FOLR2+ macrophages promotes fibrosis in chronic kidney disease
Nature Communications (2024)
-
SDMA attenuates renal tubulointerstitial fibrosis through inhibition of STAT4
Journal of Translational Medicine (2023)
-
C3a/C3aR synergies with TGF-β to promote epithelial-mesenchymal transition of renal tubular epithelial cells via the activation of the NLRP3 inflammasome
Journal of Translational Medicine (2023)
-
Enhancer of zeste homolog 2 promotes renal fibrosis after acute kidney injury by inducing epithelial-mesenchymal transition and activation of M2 macrophage polarization
Cell Death & Disease (2023)
-
Knockdown of lncRNA MALAT1 attenuates renal interstitial fibrosis through miR-124-3p/ITGB1 axis
Scientific Reports (2023)