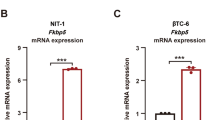
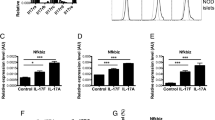
Abstract
In type 2 diabetes, hyperglycemia is present when an increased demand for insulin, typically due to insulin resistance, is not met as a result of progressive pancreatic beta cell dysfunction. This defect in beta cell activity is typically characterized by impaired insulin biosynthesis and secretion, usually accompanied by oxidative and endoplasmic reticulum (ER) stress. We demonstrate that multiple inflammatory cytokines elevated in diabetic pancreatic islets induce beta cell oxidative and ER stress, with interleukin-23 (IL-23), IL-24 and IL-33 being the most potent. Conversely, we show that islet-endogenous and exogenous IL-22, by regulating oxidative stress pathways, suppresses oxidative and ER stress caused by cytokines or glucolipotoxicity in mouse and human beta cells. In obese mice, antibody neutralization of IL-23 or IL-24 partially reduced beta cell ER stress and improved glucose tolerance, whereas IL-22 administration modulated oxidative stress regulatory genes in islets, suppressed ER stress and inflammation, promoted secretion of high-quality efficacious insulin and fully restored glucose homeostasis followed by restitution of insulin sensitivity. Thus, therapeutic manipulation of immune regulators of beta cell stress reverses the hyperglycemia central to diabetes pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ashcroft, F.M. & Rorsman, P. Diabetes mellitus and the beta cell: the last ten years. Cell 148, 1160–1171 (2012).
Back, S.H. & Kaufman, R.J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 81, 767–793 (2012).
Cnop, M., Foufelle, F. & Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 18, 59–68 (2012).
Donath, M.Y. & Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Volchuk, A. & Ron, D. The endoplasmic reticulum stress response in the pancreatic beta-cell. Diabetes Obes. Metab. 12 (suppl. 2), 48–57 (2010).
Fonseca, S.G., Urano, F., Weir, G.C., Gromada, J. & Burcin, M. Wolfram syndrome 1 and adenylyl cyclase 8 interact at the plasma membrane to regulate insulin production and secretion. Nat. Cell Biol. 14, 1105–1112 (2012).
Hasnain, S.Z., Lourie, R., Das, I., Chen, A.C. & McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 90, 260–270 (2012).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Chan, J.Y., Biden, T.J. & Laybutt, D.R. Cross-talk between the unfolded protein response and nuclear factor-κB signalling pathways regulates cytokine-mediated beta cell death in MIN6 cells and isolated mouse islets. Diabetologia 55, 2999–3009 (2012).
Kacheva, S., Lenzen, S. & Gurgul-Convey, E. Differential effects of proinflammatory cytokines on cell death and ER stress in insulin-secreting INS1E cells and the involvement of nitric oxide. Cytokine 55, 195–201 (2011).
Gurzov, E.N. et al. Signaling by IL-1β+IFN-γ and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ. 16, 1539–1550 (2009).
Ghosh, R. et al. Allosteric Inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158, 534–548 (2014).
Lee, A.H., Heidtman, K., Hotamisligil, G.S. & Glimcher, L.H. Dual and opposing roles of the unfolded protein response regulated by IRE1α and XBP1 in proinsulin processing and insulin secretion. Proc. Natl. Acad. Sci. USA 108, 8885–8890 (2011).
Oslowski, C.M. et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 16, 265–273 (2012).
Lerner, A.G. et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264 (2012).
Chen, J., Fontes, G., Saxena, G., Poitout, V. & Shalev, A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid–induced ER stress–mediated beta-cell death. Diabetes 59, 440–447 (2010).
Larsen, C.M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Moran, A. et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 381, 1905–1915 (2013).
Rissanen, A., Howard, C.P., Botha, J. & Thuren, T. Effect of anti-IL-1β antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes. Metab. 14, 1088–1096 (2012).
Ridker, P.M. et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 126, 2739–2748 (2012).
Cavelti-Weder, C. et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care 35, 1654–1662 (2012).
Sloan-Lancaster, J. et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Diabetes Care 36, 2239–2246 (2013).
Iwawaki, T., Akai, R., Kohno, K. & Miura, M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 10, 98–102 (2004).
Cunha, D.A. et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J. Cell Sci. 121, 2308–2318 (2008).
Shioya, M., Andoh, A., Kakinoki, S., Nishida, A. & Fujiyama, Y. Interleukin 22 receptor 1 expression in pancreas islets. Pancreas 36, 197–199 (2008).
Harding, H.P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Mahdi, T. et al. Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab. 16, 625–633 (2012).
Cobleigh, M.A. & Robek, M.D. Protective and pathological properties of IL-22 in liver disease: implications for viral hepatitis. Am. J. Pathol. 182, 21–28 (2013).
Malhi, H. & Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 54, 795–809 (2011).
Wang, X. et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 10.1038/nature13564 (6 August 2014).
Igoillo-Esteve, M. et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia 53, 1395–1405 (2010).
Kaneko, M., Niinuma, Y. & Nomura, Y. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 26, 931–935 (2003).
Pahl, H.L. & Baeuerle, P.A. Activation of NF-κB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 392, 129–136 (1996).
Xue, J., Nguyen, D.T. & Habtezion, A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology 143, 1670–1680 (2012).
Papa, F.R. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb. Perspect. Med. 2, a007666 (2012).
Newsholme, P. et al. Reactive oxygen and nitrogen species generation, antioxidant defenses, and beta-cell function: a critical role for amino acids. J. Endocrinol. 214, 11–20 (2012).
Malhotra, J.D. & Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9, 2277–2293 (2007).
Cardozo, A.K. et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54, 452–461 (2005).
Bedoya, F.J. et al. Regulation of pancreatic beta-cell survival by nitric oxide: clinical relevance. Islets 4, 108–118 (2012).
Dickhout, J.G. et al. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 25, 2623–2629 (2005).
Hou, R. et al. Upregulation of PTEN by peroxynitrite contributes to cytokine-induced apoptosis in pancreatic beta-cells. Apoptosis 15, 877–886 (2010).
Knoops, B., Goemaere, J., Van der Eecken, V. & Declercq, J.P. Peroxiredoxin 5: structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid. Redox Signal. 15, 817–829 (2011).
Zhang, H.M., Dang, H., Yeh, C.K. & Zhang, B.X. Linoleic acid-induced mitochondrial Ca2+ efflux causes peroxynitrite generation and protein nitrotyrosylation. PLoS ONE 4, e6048 (2009).
Bryan, H.K., Olayanju, A., Goldring, C.E. & Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 85, 705–717 (2013).
Dalmas, E. et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes 63, 1966–1977 (2014).
Yang, L. et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J. Hepatol. 53, 339–347 (2010).
Akiyama, M. et al. X-box binding protein 1 is essential for insulin regulation of pancreatic alpha cell function. Diabetes 62, 2439–2449 (2013).
Walker, J.N. et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes. Metab. 13 (suppl. 1), 95–105 (2011).
Upadhyay, V. et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat. Immunol. 13, 947–953 (2012).
Hasnain, S.Z. et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 144, 357–368 (2013).
Wei, X. et al. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe 11, 140–152 (2012).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Fabbrini, E. et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 145, 366–374 e1–3 (2013).
Zhao, R. et al. Elevated peripheral frequencies of TH22 cells: a novel potent participant in obesity and type 2 diabetes. PLoS ONE 9, e85770 (2014).
Wang, Z. et al. High fat diet induces formation of spontaneous liposarcoma in mouse adipose tissue with overexpression of interleukin 22. PLoS ONE 6, e23737 (2011).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23–induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Kirchberger, S. et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210, 917–931 (2013).
Marhfour, I. et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 55, 2417–2420 (2012).
Tersey, S.A. et al. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61, 818–827 (2012).
Miyazaki, J. et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127, 126–132 (1990).
Sakon, S. et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 22, 3898–3909 (2003).
Zhang, J. et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 7, 1068–1085 (2012).
Coughlan, M.T. et al. Advanced glycation end products are direct modulators of beta-cell function. Diabetes 60, 2523–2532 (2011).
Ricordi, C., Lacy, P.E. & Scharp, D.W. Automated islet isolation from human pancreas. Diabetes 38 (suppl. 1), 140–142 (1989).
Ricordi, C. et al. Islet isolation assessment in man and large animals. Acta Diabetol. Lat. 27, 185–195 (1990).
Klemm, D.J. et al. Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J. Biol. Chem. 276, 28430–28435 (2001).
Acknowledgements
We would like to thank the staff of the Mater Research and Translational Research Institute Biological Research Facilities for care of experimental animals, L. Crowley and S. Roy for assistance with confocal microscopy, H. Nielsen for technical assistance with immunofluorescence staining, and A. Bertolotti and D. Serisier for discussions about the manuscript. J.M.F., J.P.W. and M.A.M. are or were supported by Australian National Health and Medical Research Council Senior Research Fellowships. The research was supported by Australian National Health and Medical Research Council Project Grant 1047905 and the Mater Foundation. MIN6N8 cells were a kind gift from J. Miyazaki, Osaka University. F-XBP1ΔDBD-venus was a kind gift from M. Miura, University of Tokyo. Anti–IL-23 was a gift from Eli Lilly.
Author information
Authors and Affiliations
Contributions
S.Z.H., J.M.F., J.P.W., J.B.P. and M.A.M. developed the concept, designed the experiments, interpreted the data and wrote the manuscript. S.Z.H. was involved in all the experimental work and data analysis. D.J.B. conducted studies with mouse islets. B.E.H. conducted the studies in db/db mice. H.T., C.P.N., I.D., R.W. and A.C.-H.C. conducted experiments in HFD mice. T.L., T.W.K. and H.E.T. isolated human islets and contributed to experiments with human islets. Y.H.S. conducted flow cytometry experiments. B.E.H., D.J.B., H.T., Y.H.S., C.P.N., I.D., R.W., A.C.-H.C., T.L., T.W.K. and H.E.T. contributed to redrafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–5. (PDF 1731 kb)
DHE activation in vehicle control–treated cells.
MIN6N8 cells were loaded with dihydroethidium (DHE) which fluoresces and binds DNA after oxidation by O2 – and the nuclear dye DAPI. Cells were exposed to vehicle only 50 ng mL–1 IL-23 for 30 min ± 50 ng mL–1 IL-22 at the same time or 30 min prior to IL-23. Live cell imaging in MIN6N8 cells was carried out at 37 °C in 5% CO2 in a chamber using an Olympus xcellence rt microscope. Time-lapse imaging (three frames/min for 30 min) was utilized to determine DHEconversion/ fluorescence in cells after treatment. DAPI staining was captured to visualize nuclei. The original TIFF images were compressed into JPG format to prepare the videos to reduce file size. Individual experiment conducted in a single plate at the same time. (MP4 1174 kb)
DHE activation in IL-23–treated cells.
MIN6N8 cells were loaded with dihydroethidium (DHE) which fluoresces and binds DNA after oxidation by O2 – and the nuclear dye DAPI. Cells were exposed to vehicle only 50 ng mL–1 IL-23 for 30 min ± 50 ng mL–1 IL–22 at the same time or 30 min prior to IL-23. Live cell imaging in MIN6N8 cells was carried out at 37 °C in 5% CO2 in a chamber using an Olympus xcellence rt microscope. Time-lapse imaging (three frames/min for 30 min) was utilized to determine DHEconversion/ fluorescence in cells after treatment. DAPI staining was captured to visualize nuclei. The original TIFF images were compressed into JPG format to prepare the videos to reduce file size. Individual experiment conducted in a single plate at the same time. (MP4 6486 kb)
DHE activation in cells concomitantly treated with IL-23 and IL-22.
MIN6N8 cells were loaded with dihydroethidium (DHE) which fluoresces and binds DNA after oxidation by O2 – and the nuclear dye DAPI. Cells were exposed to vehicle only 50 ng mL–1 IL-23 for 30 min ± 50 ng mL–1 IL-22 at the same time or 30 min prior to IL-23. Live cell imaging in MIN6N8 cells was carried out at 37 °C in 5% CO2 in a chamber using an Olympus xcellence rt microscope. Time-lapse imaging (three frames/min for 30 min) was utilized to determine DHEconversion/ fluorescence in cells after treatment. DAPI staining was captured to visualize nuclei. The original TIFF images were compressed into JPG format to prepare the videos to reduce file size. Individual experiment conducted in a single plate at the same time. (MP4 21777 kb)
DHE activation in cells treated with IL-23 following a 30 min pretreatment with IL-22.
MIN6N8 cells were loaded with dihydroethidium (DHE) which fluoresces and binds DNA after oxidation by O2 – and the nuclear dye DAPI. Cells were exposed to vehicle only 50 ng mL–1 IL-23 for 30 min ± 50 ng mL–1 IL-22 at the same time or 30 min prior to IL-23. Live cell imaging in MIN6N8 cells was carried out at 37 °C in 5% CO2 in a chamber using an Olympus xcellence rt microscope. Time-lapse imaging (three frames/min for 30 min) was utilized to determine DHEconversion/ fluorescence in cells after treatment. DAPI staining was captured to visualize nuclei. The original TIFF images were compressed into JPG format to prepare the videos to reduce file size. Individual experiment conducted in a single plate at the same time. (MP4 665 kb)
Rights and permissions
About this article
Cite this article
Hasnain, S., Borg, D., Harcourt, B. et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 20, 1417–1426 (2014). https://doi.org/10.1038/nm.3705
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3705
This article is cited by
-
Opportunities and challenges: interleukin-22 comprehensively regulates polycystic ovary syndrome from metabolic and immune aspects
Journal of Ovarian Research (2023)
-
The NERP-4–SNAT2 axis regulates pancreatic β-cell maintenance and function
Nature Communications (2023)
-
Interleukin-22/Interleukin-22 binding protein axis and oral contraceptive use in polycystic ovary syndrome
Endocrine (2023)
-
Metabolic control of innate lymphoid cells in health and disease
Nature Metabolism (2022)
-
Human plasma proteomic profiles indicative of cardiorespiratory fitness
Nature Metabolism (2021)