Abstract
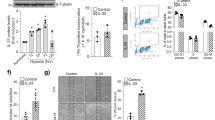
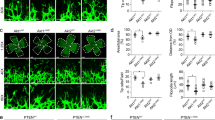
The ataxia telangiectasia mutated (ATM) kinase, a master regulator of the DNA damage response (DDR), acts as a barrier to cellular senescence and tumorigenesis. Aside from DDR signaling, ATM also functions in oxidative defense. Here we show that Atm in mice is activated specifically in immature vessels in response to the accumulation of reactive oxygen species (ROS). Global or endothelial-specific Atm deficiency in mice blocked pathological neoangiogenesis in the retina. This block resulted from increased amounts of ROS and excessive activation of the mitogen activated kinase p38α rather than from defects in the canonical DDR pathway. Atm deficiency also lowered tumor angiogenesis and enhanced the antiangiogenic action of vascular endothelial growth factor (Vegf) blockade. These data suggest that pathological neoangiogenesis requires ATM-mediated oxidative defense and that agents that promote excessive ROS generation may have beneficial effects in the treatment of neovascular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ferrara, N. VEGF-A: a critical regulator of blood vessel growth. Eur. Cytokine Netw. 20, 158–163 (2009).
Carmeliet, P. & Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Ratner, M. Genentech discloses safety concerns over Avastin. Nat. Biotechnol. 22, 1198 (2004).
Kamba, T. & McDonald, D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 96, 1788–1795 (2007).
Bergers, G. & Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 8, 592–603 (2008).
Weis, S.M. & Cheresh, D.A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17, 1359–1370 (2011).
Jain, R.K. et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat. Rev. Clin. Oncol. 6, 327–338 (2009).
Saharinen, P., Eklund, L., Pulkki, K., Bono, P. & Alitalo, K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 17, 347–362 (2011).
Sedelnikova, O.A., Pilch, D.R., Redon, C. & Bonner, W.M. Histone H2AX in DNA damage and repair. Cancer Biol. Ther. 2, 233–235 (2003).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155–168 (2003).
Economopoulou, M. et al. Histone H2AX is integral to hypoxia-driven neovascularization. Nat. Med. 15, 553–558 (2009).
Zhan, H., Suzuki, T., Aizawa, K., Miyagawa, K. & Nagai, R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J. Biol. Chem. 285, 29662–29670 (2010).
Boder, E. & Sedgwick, R.P. Ataxia-telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics 21, 526–554 (1958).
Raz-Prag, D. et al. A role for vascular deficiency in retinal pathology in a mouse model of ataxia-telangiectasia. Am. J. Pathol. 179, 1533–1541 (2011).
Bakkenist, C.J. & Kastan, M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
Smith, L.E. et al. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 35, 101–111 (1994).
Connor, K.M. et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4, 1565–1573 (2009).
Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Lange, C. et al. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefes Arch. Clin. Exp. Ophthalmol. 247, 1205–1211 (2009).
Dorrell, M.I. et al. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J. Clin. Invest. 119, 611–623 (2009).
Kubota, Y. et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med. 206, 1089–1102 (2009).
Fukushima, Y. et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Invest. 121, 1974–1985 (2011).
Clausen, B.E., Burkhardt, C., Reith, W., Renkawitz, R. & Förster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Marquardt, T. et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55 (2001).
Lau, A. et al. Suppression of HIV-1 infection by a small molecule inhibitor of the ATM kinase. Nat. Cell Biol. 7, 493–500 (2005).
Sawamiphak, S. et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465, 487–491 (2010).
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12, 446–451 (2006).
Barlow, C. et al. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc. Natl. Acad. Sci. USA 96, 9915–9919 (1999).
Barzilai, A., Rotman, G. & Shiloh, Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst.) 1, 3–25 (2002).
McKinnon, P.J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu. Rev. Pathol. 7, 303–321 (2012).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 (2009).
Iwasa, H., Han, J. & Ishikawa, F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells 8, 131–144 (2003).
Tourian, L. Jr., Zhao, H. & Srikant, C.B. p38α, but not p38β, inhibits the phosphorylation and presence of c-FLIPS in DISC to potentiate Fas-mediated caspase-8 activation and type I apoptotic signaling. J. Cell Sci. 117, 6459–6471 (2004).
Hida, K. & Klagsbrun, M. A new perspective on tumor endothelial cells: unexpected chromosome and centrosome abnormalities. Cancer Res. 65, 2507–2510 (2005).
Wellen, K.E. & Thompson, C.B. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol. Cell 40, 323–332 (2010).
Granger, D.N. & Korthuis, R.J. Physiologic mechanisms of postischemic tissue injury. Annu. Rev. Physiol. 57, 311–332 (1995).
Cosentino, C., Grieco, D. & Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 30, 546–555 (2011).
Xia, C. et al. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 67, 10823–10830 (2007).
Dong, A. et al. Oxidative stress promotes ocular neovascularization. J. Cell Physiol. 219, 544–552 (2009).
West, X.Z. et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467, 972–976 (2010).
Guo, Z., Kozlov, S., Lavin, M.F., Person, M.D. & Paull, T.T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Herzog, K.H., Chong, M.J., Kapsetaki, M., Morgan, J.I. & McKinnon, P.J. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science 280, 1089–1091 (1998).
Zhang, D. et al. Cre-loxP controlled periodic Aurora-A overexpression inducesmitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene 23, 8720–8730 (2004).
Zha, S. et al. Ataxia telangiectasia-mutated protein and DNA-dependent protein kinase have complementary V(D)J recombination functions. Proc. Natl. Acad. Sci. USA 108, 2028–2033 (2011).
Nishida, K. et al. p38α mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol. Cell. Biol. 24, 10611–10620 (2004).
Pan, H. et al. Molecular targeting of antiangiogenic factor 16K hPRL inhibits oxygen-induced retinopathy in mice. Invest. Ophthalmol. Vis. Sci. 45, 2413–2419 (2004).
Acknowledgements
We thank S. Kobayashi for outstanding technical support. We also thank F.W. Alt (Howard Hughes Medical Institute, Children's Hospital, Immune Disease Institute and Harvard Medical School) for providing Atmflox/flox mice, P.J. McKinnon (St. Jude Children's Research Hospital) for providing Atm−/− mice and H. Saya (Division of Gene Regulation, Keio University) for providing p53−/− mice. This work was supported by Grants-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by a research grant from Takeda Science Foundation, by the AstraZeneca Virtual Research Institute Research Grant and by the Keio Kanrinmaru Project.
Author information
Authors and Affiliations
Contributions
Y.O. and A.N.-I. performed experiments and analyzed data. T.S. interpreted results and assisted in manuscript preparation. K.O. provided experimental materials. Y.K. designed experiments, interpreted results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 (PDF 4179 kb)
Rights and permissions
About this article
Cite this article
Okuno, Y., Nakamura-Ishizu, A., Otsu, K. et al. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med 18, 1208–1216 (2012). https://doi.org/10.1038/nm.2846
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2846
This article is cited by
-
Beyond starving cancer: anti-angiogenic therapy
Journal of Medical Ultrasonics (2023)
-
STAT3 and PD-L1 are negatively correlated with ATM and have impact on the prognosis of triple-negative breast cancer patients with low ATM expression
Breast Cancer Research and Treatment (2022)
-
Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism
Cell Death & Differentiation (2020)
-
Functional interplay between the oxidative stress response and DNA damage checkpoint signaling for genome maintenance in aerobic organisms
Journal of Microbiology (2020)
-
Effects of bradykinin on the survival of multiterritory perforator flaps in rats
World Journal of Surgical Oncology (2019)