Abstract
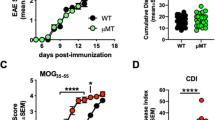
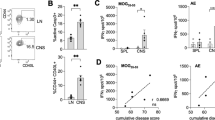
We describe here the paradoxical development of spontaneous experimental autoimmune encephalomyelitis (EAE) in transgenic mice expressing a myelin oligodendrocyte glycoprotein (MOG)-specific T cell antigen receptor (TCR) in the absence of MOG. We report that in Mog-deficient mice (Mog−/−), the autoimmune response by transgenic T cells is redirected to a neuronal cytoskeletal self antigen, neurofilament-M (NF-M). Although components of radically different protein classes, the cross-reacting major histocompatibility complex I-Ab–restricted epitope sequences of MOG35–55 and NF-M18–30 share essential TCR contact positions. This pattern of cross-reaction is not specific to the transgenic TCR but is also commonly seen in MOG35–55–I-Ab–reactive T cells. We propose that in the C57BL/6 mouse, MOG and NF-M response components add up to overcome the general resistance of this strain to experimental induction of autoimmunity. Similar cumulative responses against more than one autoantigen may have a role in spontaneously developing human autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Goebels, N. et al. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects. Epitope spreading versus clonal persistence. Brain 123, 508–518 (2000).
Wekerle, H. Breaking ignorance: the case of the brain. in Current Concepts in Autoimmunity and Chronic Inflammation (eds. Radbruch, A. & Lipsky, P.E.) 25–50 (Springer, Berlin, 2006).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein–specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Litzenburger, T. et al. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J. Exp. Med. 188, 169–180 (1998).
Delarasse, C. et al. Myelin/oligodendrocyte glycoprotein–deficient (MOG-deficient) mice reveal lack of immune tolerance to MOG in wild-type mice. J. Clin. Invest. 112, 544–553 (2003).
Krishnamoorthy, G., Lassmann, H., Wekerle, H. & Holz, A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J. Clin. Invest. 116, 2385–2392 (2006).
Bettelli, E., Baeten, D., Jäger, A., Sobel, R.A. & Kuchroo, V.K. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J. Clin. Invest. 116, 2393–2402 (2006).
Hövelmeyer, N. et al. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J. Immunol. 175, 5875–5884 (2005).
Norton, W.T. & Poduslo, S.E. Myelination in rat brain changes in myelin composition during maturation. J. Neurochem. 21, 759–773 (1973).
Chantry, A., Gregson, N.A. & Glynn, P. A novel metalloproteinase associated with brain myelin membranes. Isolation and characterization. J. Biol. Chem. 264, 21603–21607 (1989).
Petersen, T.R. et al. Characterization of MHC- and TCR-binding residues of the myelin oligodendrocyte glycoprotein 38–51 peptide. Eur. J. Immunol. 34, 165–173 (2004).
Ben-Nun, A. et al. Anatomy of T cell autoimmunity to myelin oligodendrocyte glycoprotein (MOG): prime role of MOG44F in selection and control of MOG-reactive T cells in H-2b mice. Eur. J. Immunol. 36, 478–493 (2006).
Mendel, I., Kerlero de Rosbo, N. & Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor Vβ expression of encephalitogenic T cells. Eur. J. Immunol. 25, 1951–1959 (1995).
Wucherpfennig, K.W. & Strominger, J.L. Molecular mimicry in T cell–mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80, 695–705 (1995).
Kelly, B.M., Gillespie, C.S., Sherman, D.L. & Brophy, P.J. Schwann cells of the myelin-forming phenotype express neurofilament protein NF-M. J. Cell Biol. 118, 397–410 (1992).
Huizinga, R. et al. Immunization with neurofilament light protein induces spastic paresis and axonal degeneration in Biozzi ABH mice. J. Neuropathol. Exp. Neurol. 66, 295–304 (2007).
Bartos, A. et al. Elevated intrathecal antibodies against the medium neurofilament subunit in multiple sclerosis. J. Neurol. 254, 20–25 (2007).
Berger, T. et al. Experimental autoimmune encephalomyelitis: the antigen specificity of T-lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab. Invest. 76, 355–364 (1997).
Kojima, K. et al. Experimental autoimmune panencephalitis and uveoretinitis in the Lewis rat transferred by T lymphocytes specific for the S100β molecule, a calcium binding protein of astroglia. J. Exp. Med. 180, 817–829 (1994).
Abromson-Leeman, S. et al. T cell responses to myelin basic protein in experimental autoimmune encephalomyelitis-resistant BALB/c mice. J. Neuroimmunol. 45, 89–101 (1993).
Stefferl, A. et al. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J. Immunol. 165, 2859–2865 (2000).
Jacomy, H., Zhu, Q.Z., Couillard-Despres, S., Beaulieu, J.M. & Julien, J.P. Disruption of type IV intermediate filament network in mice lacking the neurofilament medium and heavy subunits. J. Neurochem. 73, 972–984 (1999).
Amor, S. et al. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J. Immunol. 153, 4349–4356 (1994).
Acknowledgements
MogCre/Cre, Nefm−/−, 2D2 and Mog−/− mice were generously provided by A. Waisman (Johannes Gutenberg University of Mainz), J.-P. Julien (Laval University), V.K. Kuchroo (Harvard Medical School) and D. Pham-Dinh (INSERM UMR 546). We thank F. Lottspeich for granting us permission to use his mass spectrometer. We thank L. Penner and I. Arnold-Ammer for technical support. This project was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereiche (SFB) 571, Projects A1 and B6) and the Max Planck Society. H.S.D. is supported by a PhD fellowship (Portuguese Fundação para a Ciência ea Tecnologia (FCT) program SFRH/BD/15885/2005). Part of the study (conducted by H.W., R.L. and H.L.) was funded by the EU Project Neuropromise (PL 018637), and A.B.-N. was supported by the Israel Science Foundation and the National Multiple Sclerosis Society of New York (RG3195B8/2). A.B.-N. is an Alexander von Humboldt Prize Awardee.
Author information
Authors and Affiliations
Contributions
G.K. performed most of the experiments. G.K. and H.W. designed the study and wrote the manuscript with input from co-authors. A.S., L.T.M. and R.S.L. contributed EAE and T cell data. K.D. supervised protein purification and mass spectrometry and performed in silico searches. R.M. performed mass spectrometry. H.S.D. assisted in EAE experiments. A.B.-N. performed T cell line transfer EAE experiments. H.L. performed and interpreted histology. F.C.K. designed experiments and performed protein purification.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–13, Supplementary Tables 1 and 2 and Supplementary Methods (PDF 1029 kb)
Supplementary Video 1
Healthy 2D2 mouse. (MOV 2391 kb)
Supplementary Video 2
2D2 mouse with hind limb clasping phenotype. (MOV 2152 kb)
Supplementary Video 3
2D2 mouse with hind limb hyperextension (spasticity). (MOV 3284 kb)
Supplementary Video 4
Healthy 2D2 × Mog−/− mouse. (MOV 4442 kb)
Supplementary Video 5
2D2 × Mog−/− mouse with hind limb clasping. (MOV 3968 kb)
Supplementary Video 6
2D2 × Mog−/− mouse with hind limb clasping and hyperextension (spasticity). (MOV 3087 kb)
Rights and permissions
About this article
Cite this article
Krishnamoorthy, G., Saxena, A., Mars, L. et al. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat Med 15, 626–632 (2009). https://doi.org/10.1038/nm.1975
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1975
This article is cited by
-
Cholinergic control of Th17 cell pathogenicity in experimental autoimmune encephalomyelitis
Cell Death & Differentiation (2023)
-
A GM-CSF-neuroantigen tolerogenic vaccine elicits inefficient antigen recognition events below the CD40L triggering threshold to expand CD4+ CD25+ FOXP3+ Tregs that inhibit experimental autoimmune encephalomyelitis (EAE)
Journal of Neuroinflammation (2020)
-
Deciphering CD4+ T cell specificity using novel MHC–TCR chimeric receptors
Nature Immunology (2019)
-
β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration
Nature (2019)
-
T cell responses in the central nervous system
Nature Reviews Immunology (2017)