Abstract
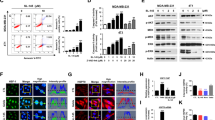
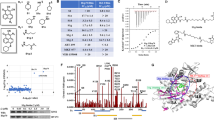
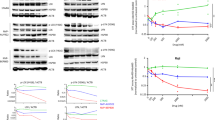
We report that heat shock protein 90 (Hsp90) inhibitors selectively kill diffuse large B cell lymphomas (DLBCLs) that depend on the BCL-6 transcriptional repressor. We found that endogenous Hsp90 interacts with BCL-6 in DLBCL cells and can stabilize BCL-6 mRNA and protein. Hsp90 formed a complex with BCL-6 at its target promoters, and Hsp90 inhibitors derepressed BCL-6 target genes. A stable mutant of BCL-6 rescued DLBCL cells from Hsp90 inhibitor–induced apoptosis. BCL-6 and Hsp90 were almost invariantly coexpressed in the nuclei of primary DLBCL cells, suggesting that their interaction is relevant in this disease. We examined the pharmacokinetics, toxicity and efficacy of PU-H71, a recently developed purine-derived Hsp90 inhibitor. PU-H71 preferentially accumulated in lymphomas compared to normal tissues and selectively suppressed BCL-6–dependent DLBCLs in vivo, inducing reactivation of key BCL-6 target genes and apoptosis. PU-H71 also induced cell death in primary human DLBCL specimens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
03 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41591-024-02957-0
References
Neckers, L. Heat shock protein 90: the cancer chaperone. J. Biosci. 32, 517–530 (2007).
Wandinger, S.K., Richter, K. & Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 283, 18473–18477 (2008).
Bonvini, P., Gastaldi, T., Falini, B. & Rosolen, A. Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK+ CD30+ lymphoma cells by the Hsp90 antagonist 17-allylamino,17-demethoxygeldanamycin. Cancer Res. 62, 1559–1566 (2002).
Nimmanapalli, R., O'Bryan, E. & Bhalla, K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl–positive human leukemic blasts. Cancer Res. 61, 1799–1804 (2001).
Chiosis, G. et al. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem. Biol. 8, 289–299 (2001).
Caldas-Lopes, E. et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc. Natl. Acad. Sci. USA 106, 8368–8373 (2009).
Taldone, T., Gozman, A., Maharaj, R. & Chiosis, G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr. Opin. Pharmacol. 8, 373–374 (2008).
Chiosis, G. Discovery and development of purine-scaffold Hsp90 inhibitors. Curr. Top. Med. Chem. 6, 1183–1191 (2006).
Chiosis, G. et al. Development of purine-scaffold small molecule inhibitors of Hsp90. Curr. Cancer Drug Targets 3, 371–376 (2003).
Chiosis, G., Lucas, B., Shtil, A., Huezo, H. & Rosen, N. Development of a purine-scaffold novel class of Hsp90 binders that inhibit the proliferation of cancer cells and induce the degradation of Her2 tyrosine kinase. Bioorg. Med. Chem. 10, 3555–3564 (2002).
He, H. et al. Identification of potent water soluble purine-scaffold inhibitors of the heat shock protein 90. J. Med. Chem. 49, 381–390 (2006).
Chiosis, G., Rodina, A. & Moulick, K. Emerging Hsp90 inhibitors: from discovery to clinic. Anticancer. Agents Med. Chem. 6, 1–8 (2006).
Klein, U. & Dalla-Favera, R. Germinal centres: role in B cell physiology and malignancy. Nat. Rev. Immunol. 8, 22–33 (2008).
Ci, W., Polo, J.M. & Melnick, A. B cell lymphoma 6 and the molecular pathogenesis of diffuse large B cell lymphoma. Curr. Opin. Hematol. 15, 381–390 (2008).
Phan, R.T. & Dalla-Favera, R. The BCL-6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature 432, 635–639 (2004).
Ranuncolo, S.M. et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat. Immunol. 8, 705–714 (2007).
Cerchietti, L.C. et al. A peptomimetic inhibitor of BCL-6 with potent antilymphoma effects in vitro and in vivo. Blood 113, 3397–3405 (2009).
Polo, J.M. et al. Specific peptide interference reveals BCL-6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat. Med. 10, 1329–1335 (2004).
Valbuena, J.R. et al. Expression of heat-shock protein-90 in non-Hodgkin's lymphomas. Mod. Pathol. 18, 1343–1349 (2005).
Monti, S. et al. Molecular profiling of diffuse large B cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 105, 1851–1861 (2005).
Polo, J.M. et al. Transcriptional signature with differential expression of BCL-6 target genes accurately identifies BCL-6–dependent diffuse large B cell lymphomas. Proc. Natl. Acad. Sci. USA 104, 3207–3212 (2007).
Schulte, T.W., Blagosklonny, M.V., Ingui, C. & Neckers, L. Disruption of the Raf-1–Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J. Biol. Chem. 270, 24585–24588 (1995).
Sato, S., Fujita, N. & Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 97, 10832–10837 (2000).
Broemer, M., Krappmann, D. & Scheidereit, C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene 23, 5378–5386 (2004).
Niu, H., Ye, B.H. & Dalla-Favera, R. Antigen receptor signaling induces MAP kinase–mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 12, 1953–1961 (1998).
Laroia, G., Cuesta, R., Brewer, G. & Schneider, R.J. Control of mRNA decay by heat shock–ubiquitin-proteasome pathway. Science 284, 499–502 (1999).
Wax, S., Piecyk, M., Maritim, B. & Anderson, P. Geldanamycin inhibits the production of inflammatory cytokines in activated macrophages by reducing the stability and translation of cytokine transcripts. Arthritis Rheum. 48, 541–550 (2003).
Sinsimer, K.S. et al. Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element–mediated mRNA decay. Mol. Cell. Biol. 28, 5223–5237 (2008).
Dean, J.L., Sully, G., Clark, A.R. & Saklatvala, J. The involvement of AU-rich element–binding proteins in p38 mitogen-activated protein kinase pathway–mediated mRNA stabilisation. Cell. Signal. 16, 1113–1121 (2004).
Ing, N.H., Massuto, D.A. & Jaeger, L.A. Estradiol up-regulates AUF1p45 binding to stabilizing regions within the 3′-untranslated region of estrogen receptor α mRNA. J. Biol. Chem. 283, 1764–1772 (2008).
Bakheet, T., Frevel, M., Williams, B.R., Greer, W. & Khabar, K.S. ARED: human AU-rich element–containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 29, 246–254 (2001).
Liu, J. & DeFranco, D.B. Chromatin recycling of glucocorticoid receptors: implications for multiple roles of heat shock protein 90. Mol. Endocrinol. 13, 355–365 (1999).
Abu-Farha, M. et al. The tale of two domains: proteomics and genomics analysis of SMYD2, a new histone methyltransferase. Mol. Cell. Proteomics 7, 560–572 (2008).
Zhao, R. & Houry, W.A. Hsp90: a chaperone for protein folding and gene regulation. Biochem. Cell Biol. 83, 703–710 (2005).
Shinozaki, F. et al. Depletion of hsp90beta induces multiple defects in B cell receptor signaling. J. Biol. Chem. 281, 16361–16369 (2006).
Abramson, J.S. et al. The heat shock protein 90 inhibitor IPI-504 induces apoptosis of AKT-dependent diffuse large B-cell lymphomas. Br. J. Haematol. 144, 358–366 (2009).
Robles, A.I. et al. Schedule-dependent synergy between the heat shock protein 90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin and doxorubicin restores apoptosis to p53-mutant lymphoma cell lines. Clin. Cancer Res. 12, 6547–6556 (2006).
Acknowledgements
We thank J.C. Zenklusen for his contribution with gene expression profiling, D. Zatorska for the synthesis of PU-H71, L. Neckers (Urologic Oncology Branch, National Cancer Institute) for providing pGEM4Z Hsp90 vectors, V. Bardwell (University of Minnesota) for providing T7plink vectors, M. Shipp (Dana-Farber Cancer Center) for providing cell lines, and B.H. Ye (Albert Einstein College of Medicine) for providing FUW-hBCL-6 plasmid constructs and cell lines. G.C. is supported by the Geoffrey Beene Cancer Research Center of the Memorial Sloan-Kettering Cancer Center, Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Leukemia and Lymphoma Society, the Translational and Integrative Medicine Research Fund and the Experimental Therapeutics Center of the Memorial Sloan-Kettering Cancer Center. A. Melnick is supported by the Leukemia and Lymphoma Society grant S-7032-04, US National Cancer Institute grant R01-CA10434 and the Chemotherapy Foundation. This research was supported in part by the Intramural Research Program of the US National Institutes of Health National Cancer Institute Center for Cancer Research.
Author information
Authors and Affiliations
Contributions
L.C.C., G.C. and A. Melnick conceived of the project. L.C.C., E.C.L., S.N.Y., K.H., K.L.B., L.A.T., A. Mallik, A.I.R., J.W. and R.S. performed experiments and analyzed data. L.C.C., E.C.L., A. Melnick, L.V., K.N.B. and G.C. designed the studies and supervised research. K.N.B. and L.V. gave scientific advice. L.C.C., A. Melnick and G.C. wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, Supplementary Tables 1–4 and Supplementary Methods (PDF 1660 kb)
Rights and permissions
About this article
Cite this article
Cerchietti, L., Lopes, E., Yang, S. et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6–dependent B cell lymphomas. Nat Med 15, 1369–1376 (2009). https://doi.org/10.1038/nm.2059
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2059
This article is cited by
-
SIRT1 and HSP90α feed-forward circuit safeguards chromosome segregation integrity in diffuse large B cell lymphomas
Cell Death & Disease (2023)
-
Anti-dermatophytic activity of cold atmospheric plasma against Trichophyton rubrum via affecting fungal growth, morphology, drug susceptibility and HSP90 gene expression
Scientific Reports (2022)
-
Hsp90 inhibition sensitizes DLBCL cells to cisplatin
Cancer Chemotherapy and Pharmacology (2022)
-
Prostate cancer and therapeutic challenges
Journal of Biological Research-Thessaloniki (2020)
-
Inhibitors of HSP90 in melanoma
Apoptosis (2020)