Abstract
In lymph nodes, fibroblastic reticular cells (FRCs) form a collagen-based reticular network that supports migratory dendritic cells (DCs) and T cells and transports lymph. A hallmark of FRCs is their propensity to contract collagen, yet this function is poorly understood. Here we demonstrate that podoplanin (PDPN) regulates actomyosin contractility in FRCs. Under resting conditions, when FRCs are unlikely to encounter mature DCs expressing the PDPN receptor CLEC-2, PDPN endowed FRCs with contractile function and exerted tension within the reticulum. Upon inflammation, CLEC-2 on mature DCs potently attenuated PDPN-mediated contractility, which resulted in FRC relaxation and reduced tissue stiffness. Disrupting PDPN function altered the homeostasis and spacing of FRCs and T cells, which resulted in an expanded reticular network and enhanced immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Kumamoto, Y., Mattei, L.M., Sellers, S., Payne, G.W. & Iwasaki, A. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc. Natl. Acad. Sci. USA 108, 8749–8754 (2011).
Tzeng, T.C. et al. CD11chi dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes. J. Immunol. 184, 4247–4257 (2010).
Chyou, S. et al. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J. Immunol. 187, 5558–5567 (2011).
Turley, S.J., Fletcher, A.L. & Elpek, K.G. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol. 10, 813–825 (2010).
Malhotra, D., Fletcher, A.L. & Turley, S.J. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol. Rev. 251, 160–176 (2013).
Malhotra, D. et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 13, 499–510 (2012).
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 (2007).
Bajénoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Roozendaal, R. & Carroll, M.C. Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 219, 157–166 (2007).
Astarita, J.L., Acton, S.E. & Turley, S.J. Podoplanin: emerging functions in development, the immune system, and cancer. Front. Immunol. 3, 283 (2012).
Schacht, V. et al. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 22, 3546–3556 (2003).
Uhrin, P. et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 115, 3997–4005 (2010).
Wicki, A. & Christofori, G. The potential role of podoplanin in tumour invasion. Br. J. Cancer 96, 1–5 (2007).
Christou, C.M. et al. Renal cells activate the platelet receptor CLEC-2 through podoplanin. Biochem. J. 411, 133–140 (2008).
Suzuki-Inoue, K., Inoue, O. & Ozaki, Y. Novel platelet activation receptor CLEC-2: from discovery to prospects. J. Thromb. Haemost. 9, 44–55 (2011).
Acton, S.E. et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 37, 276–289 (2012).
Herzog, B.H. et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502, 105–109 (2014).
Hess, P.R. et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Invest. 124, 273–284 (2014).
Bénézech, C. et al. CLEC-2 is required for development and maintenance of lymph nodes. Blood 123, 3200–3207 (2014).
Steinman, R.M. et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann. NY Acad. Sci. 987, 15–25 (2003).
Martín-Villar, E. et al. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J. Cell Sci. 119, 4541–4553 (2006).
Riento, K. & Ridley, A.J. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4, 446–456 (2003).
Calvo, F. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637–646 (2013).
Wicki, A. et al. Tumor invasion in the absence of epithelial-mesenchymal transition: Podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 9, 261–272 (2006).
Fehon, R.G., McClatchey, A.I. & Bretscher, A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 1–12 (2010).
Larsen, M., Artym, V.V., Green, J.A. & Yamada, K.M. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463–471 (2006).
Chai, Q. et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 38, 1013–1024 (2013).
Lukacs-Kornek, V. et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 12, 1096–1104 (2011).
Siegert, S. et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS ONE 6, e27618 (2011).
Khan, O. et al. Regulation of T cell priming by lymphoid stroma. PLoS ONE 6, e26138 (2011).
Webster, B. et al. Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 203, 1903–1913 (2006).
Acton, S.E. et al. Dendritic cells control lymph node expansion via modulation of fibroblastic reticular network tension through CLEC-2/Podoplanin interactions. Nature 514, 498–502 (2014).
Cremasco, V. et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat. Immunol. 15, 973–981 (2014).
Andersson, K., Björkelund, H. & Malmqvist, M. Antibody-antigen interactions: what is the required time to equilibrium? Nature Precedings <http://hdl.handle.net/10101/npre.2010.5218.1> (2010).
Martín-Villar, E. et al. Podoplanin associates with CD44 to promote directional cell migration. Mol. Biol. Cell 21, 4387–4399 (2010).
Cueni, L.N. & Detmar, M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp. Cell Res. 315, 1715–1723 (2009).
Nakazawa, Y. et al. Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood 112, 1730–1739 (2008).
Fernández-Muñoz, B. et al. The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial-mesenchymal transition. Int. J. Biochem. Cell Biol. 43, 886–896 (2011).
Midgley, A.C. et al. Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 288, 14824–14838 (2013).
Krishnan, H. et al. Serines in the intracellular tail of podoplanin (PDPN) regulate cell motility. J. Biol. Chem. 288, 12215–12221 (2013).
Navarro, A., Perez, R.E., Rezaiekhaligh, M., Mabry, S.M. & Ekekezie, I.I. T1 /podoplanin is essential for capillary morphogenesis in lymphatic endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L543–L551 (2008).
Paszek, M.J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Klein, E.A. et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr. Biol. 19, 1511–1518 (2009).
Xia, H., Nho, R.S., Kahm, J., Kleidon, J. & Henke, C.A. Focal dhesion kinase Is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a β1 integrin viability signaling pathway. J. Biol. Chem. 279, 33024–33034 (2004).
Yang, C.Y. et al. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc. Natl. Acad. Sci. USA 111, E109–E118 (2014).
Peduto, L. et al. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J. Immunol. 182, 5789–5799 (2009).
Fletcher, A.L. et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front. Immunol. 2, 35–50 (2011).
Lamprecht, M., Sabatini, D. & Carpenter, A. CellProfiler™: free, versatile software for automated biological image analysis. Biotechniques 42, 71–75 (2007).
Acknowledgements
We thank L. Cameron for assistance at the Dana-Farber Cancer Institute confocal microscopy core, and R. Gelman for help with statistical analyses. Supported by the US National Institutes of Health (R0I DK074500, P01 AI045757 and R21 CA182598 to S.J.T.; 5R01 AI039246 to M.C.C.; and P01 HL085607 and GM103441 to L.X.), the American Cancer Society (S.J.T.), the Claudia Adams Barr Program in Innovative Cancer Research (S.J.T.), the Cancer Research Institute (to V.C.), the American Heart Association (SDG7410022 to J.F.) and the National Science Foundation (J.L.A.).
Author information
Authors and Affiliations
Contributions
J.L.A. and S.J.T. designed experiments, analyzed results and wrote the manuscript; J.L.A., V.C., J.F., M.C.D., J.R.P., J.M.N.-B., A.G., M.C.W. and R.M.W. performed experiments; J.F., K.S., Y.K., and L.X. supplied reagents and mice; L.O. and B.L. provided mice; and V.C., B.L., M.C.C., D.J.M. and L.X. provided comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.J.T., A.G. and R.M.W. are employees of Genentech.
Integrated supplementary information
Supplementary Figure 1 PDPN is required for cell adhesion and contraction.
(a) Representative histograms of FRC surface markers on wild-type and Pdpn−/− FRCs. (b) Graph depicting the area covered by FRCs as they spread on collagen at different time points after plating. (c) Graph representing the absorbance of crystal violet, which was used to stain FRCs adhering to collagen-coated wells at different time points after plating. (d-f). Wild-type FRCs were seeded into 3D collagen gels and then treated with the ROCK inhibitor Y27632. FRC morphology index (d) and the length (e) and number (f) of protrusions were measured. Data are representative of 3 independent experiments (mean±s.d., n>50 cells per condition per experiment). (g) Representative image of YAP staining in wild-type and Pdpn−/− FRCs. Data are representative of 3 independent experiments. NS, not significant *P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001 (Mann Whitney test).
Supplementary Figure 2 Generation of Δcyto mice.
(a) Schematic indicating the gene targeting strategy for the generation of the Δcyto mice. (b) Sequences of PCR fragments amplified from DNA from tail biopsies of wild-type and Δcyto pups indicating stop codons introduced after the transmembrane domain. (c) Representative histogram indicating surface levels of PDPN on wild-type, Pdpn−/−, and cyto FRCs. (d) Representative confocal images of PDPN staining in FRCs. Scale bar, 20 μm. (e) Immunoblot showing total levels of PDPN on wild-type, Pdpn−/−, and cyto FRCs. (f) Wild-type and Δcyto FRCs were treated with phosphatidylinositol-specific phospholipase C (PI-PLC) for 1 h at 37°C and the levels of Thy1 (a known GPI-anchored protein) or PDPN were measured by flow cytometry. (g) Representative image of YAP staining in wild-type and Δcyto FRCs. Data are representative of 3 independent experiments.
Supplementary Figure 3 Quantification of phosphorylated ERM, phosphorylated MLC and RhoA-GTP by immunoblot analysis.
The relative densities of the bands in immunoblots for p-ERM (a), p-MLC (b), and RhoA-GTP (c) were measured and set relative to those of wild-type FRCs. Graphs represent 3-5 independent experiments. *P<0.001; **P<0.0001 (one sample t-test).
Supplementary Figure 4 Treatment with anti-PDPN recapitulates genetic deletion of PDPN.
(a) Morphology index of either untreated or α-PDPN-treated wild-type and Pdpn−/− FRCs. Data represent 3 independent experiments (mean±s.d., n>60 cells per experiment). (b) Graph shows the amount that antibody-treated wild-type FRCs contracted collagen gels, relative untreated FRCs. Data represent 3 independent experiments (mean±s.d., three wells per experiment). (c) Representative image of HEVs and red blood cells (Ter119) in LNs of isotype- or α-PDPN-treated mice. (d-g) Graphs depicting the levels of the inflammatory markers IL-6 (d), neutrophil myeloperoxidase (MPO, e), IFNγ (f), and TNFα (g). (h) Representative flow cytometry plots indicating the forward scatter (FSC) and side scatter (SSC) profile of FRCs from LNs of mice treated with the isotype or α-PDPN antibodies. (i) Quantification of the percentage of FRCs with high FSC and SSC. Data are representative of 4 independent experiments, n=4 mice per experiment. *P<0.01; **P<0.001 (Mann Whitney test).
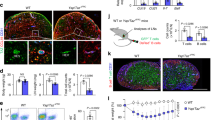
Supplementary Figure 5 Genetic deletion of PDPN results in enlarged LNs and an expanded FRC network.
(a-c) The LN masses (a), total cellularity (b), and FRC numbers (c) from Pdpnfl/fl and Pdpnfl/flPdgfrb-Cre+ mice. (d,e) Representative images of HEVs and red blood cells (Ter119) in LNs from Pdpnfl/fl and Pdpnfl/flPdgfr?-Cre+ mice (d) or wild-type and cyto mice (e). (f) Representative confocal images of conduits in isotype- and α-PDPN-treated mice that received a s.c. injection of FITC 4 h prior to imaging. FDCs are identified by CR1 staining (red) and collagen is visualized by second harmonic generation (blue). (g) Representative images of the FRC network. Confocal z-stack images of the FRC network in Pdpnfl/fl and Pdpnfl/flPdgfrb-Cre+ mice were analyzed in 3D and isosurfaces were generated. (h,i) Quantification of the surface area of desmin (h) and ER-TR7 (i) staining shown in g. (j) Representative histogram demonstrating that Ccl19-Cre+Rosa26-H2b-eGFPfl/fl mice only have a GFP signal in FRCs. (k) Representative images of FRC nuclei from Ccl19-Cre+Rosa26-H2b-eGFPfl/fl mice that were treated with the isotype or α-PDPN antibody. *P<0.05; **P<0.001; ***P<0.0001 (Mann Whitney test).
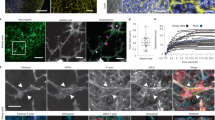
Supplementary Figure 6 Anti-PDPN does not block the binding of CLEC-2 or impair DC migration.
(a) Histogram indicating CLEC-2-Fc staining of FRCs that were either untreated, pre-treated with a hamster isotype control antibody, or pre-treated with the 8.1.1 PDPN-specific antibody. Grey line indicates the secondary alone control for the CLEC-2-Fc staining. (b,c) DC numbers (gated on PI−CD45+CD11chi) in LNs of isotype- or -PDPN-treated mice in steady state (b) or after PBS or LPS/OVA administration (c). Data are representative of 4 independent experiments (n=3-4 mice per group per experiment). *P<0.05 (Mann Whitney test).
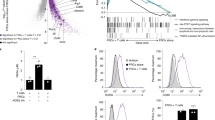
Supplementary Figure 7 CLEC-2–Fc induces elongation and impairs spreading in a way similar to a genetic deletion of PDPN.
(a) Graph depicting the morphology index for untreated or CLEC-2-Fc-treated wild-type, Pdpn−/−, and Δcyto FRCs. (b) The cell area of FRCs spreading on collagen in the presence or absence of CLEC-2-Fc. Data are representative of 3 independent experiments (mean±s.d., n>50 cells per experiment). (c) Graph representing the morphology index of wild-type FRCs that were pre-treated with cytochalasin D prior to CLEC-2-Fc treatment. (d,e) Representative images of YAP staining in wild-type (d) and Δcyto (e) FRCs that were untreated or treated with CLEC-2-Fc for 24 h. (f,g) Quantification of immunoblots for p-ERM (f) and p-MLC (g) levels in wild-type FRCs treated with CLEC-2-Fc for the indicated times. (h-k) Relative levels of inflammatory markers in LNs from mice treated with an isotype control or CLEC-2-Fc for 1 h. (l) Total cellularity from LNs of mice injected i.v. with an isotype control or CLEC-2-Fc for 1 h. (m) Morphology index for wild-type and Pdpn−/− LECs treated with an isotype control or CLEC-2-Fc for 24 h. (n) Representative images of HEVs in LNs from isotype- or CLEC-2-Fc-treated mice. Red blood cells are stained with Ter119 and can be seen leaving HEVs in CLEC-2-Fc-treated LNs. NS, not significant; *P<0.01; **P<0.001; ***P<0.0001 (Mann Whitney test).
Supplementary Figure 8 Proposed model of PDPN signaling in FRCs and LNs.
(a) LNs from both wild-type and Δcyto mice contain a dense FRC network and exhibit baseline levels of stiffness, due to maintained contraction and controlled proliferation of FRCs. (b) In wild-type FRCs, full-length PDPN associates with other transmembrane proteins to maintain high levels of ERM and MLC activation, which lead to strong adhesion to the ECM and contraction of the actomyosin skeleton within the cell. (c) Upon deletion of the cytoplasmic tail, PDPN is still able to maintain its lateral membrane interactions and localization in lipid rafts due to through its transmembrane and extracellular domains. This allows for the maintenance of ERM activation and normal contractility within the cells. In contrast, LNs from mice with treated with the α-PDPN antibody or CLEC-2-Fc (d) are enlarged and have an expanded FRC network with decreased LN stiffness. (e) In Pdpn−/− FRCs the lack of PDPN may disrupt the stability or signaling of other transmembrane binding proteins, which results in reduced ERM activation and reduced actomyosin contractility. (f) In wild-type FRCs treated with CLEC-2-Fc, ERM and MLC activation are also disrupted. While PDPN is still present in this scenario, our data suggest that CLEC-2 transiently blocks lateral membrane interactions and thus disrupts downstream signaling similarly to a complete Pdpn−/− FRC.
Supplementary information
Supplementary Figures
Supplementary Figures 1–8 (PDF 10928 kb)
Rights and permissions
About this article
Cite this article
Astarita, J., Cremasco, V., Fu, J. et al. The CLEC-2–podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol 16, 75–84 (2015). https://doi.org/10.1038/ni.3035
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3035
This article is cited by
-
Localization, tissue biology and T cell state — implications for cancer immunotherapy
Nature Reviews Immunology (2023)
-
Biomarker and genomic analyses reveal molecular signatures of non-cardioembolic ischemic stroke
Signal Transduction and Targeted Therapy (2023)
-
Mechanics drive lymph node expansion
Nature Immunology (2022)
-
Lymph node homeostasis and adaptation to immune challenge resolved by fibroblast network mechanics
Nature Immunology (2022)
-
C-type lectin-like receptor (CLEC)-2, the ligand of podoplanin, induces morphological changes in podocytes
Scientific Reports (2022)