Abstract
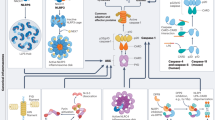
Assembly of the NLRP3 inflammasome activates caspase-1 and mediates the processing and release of the leaderless cytokine IL-1β and thereby serves a central role in the inflammatory response and in diverse human diseases. Here we found that upon activation of caspase-1, oligomeric NLRP3 inflammasome particles were released from macrophages. Recombinant oligomeric protein particles composed of the adaptor ASC or the p.D303N mutant form of NLRP3 associated with cryopyrin-associated periodic syndromes (CAPS) stimulated further activation of caspase-1 extracellularly, as well as intracellularly after phagocytosis by surrounding macrophages. We found oligomeric ASC particles in the serum of patients with active CAPS but not in that of patients with other inherited autoinflammatory diseases. Our findings support a model whereby the NLRP3 inflammasome, acting as an extracellular oligomeric complex, amplifies the inflammatory response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Lopez-Castejon, G. & Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 22, 189–195 (2011).
Dinarello, C.A., Donath, M.Y. & Mandrup-Poulsen, T. Role of IL-1β in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17, 314–321 (2010).
Neven, B. et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood 103, 2809–2815 (2004).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasomeactivation. Cell 156, 1207–1222 (2014).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Compan, V. et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37, 487–500 (2012).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857–865 (2008).
Babelova, A. et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J. Biol. Chem. 284, 24035–24048 (2009).
Yazdi, A.S. et al. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. Proc. Natl. Acad. Sci. USA 107, 19449–19454 (2010).
Eisenbarth, S.C., Colegio, O.R., O'Connor, W., Sutterwala, F.S. & Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008).
Rajamäki, K. et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 288, 13410–13419 (2013).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Lopez-Castejon, G. et al. Deubiquitinases regulate the activity of caspase-1 and interleukin-1β secretion via assembly of the inflammasome. J. Biol. Chem. 288, 2721–2733 (2013).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2009).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 109, 11282–11287 (2012).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Keller, M., Rüegg, A., Werner, S. & Beer, H.-D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Pétrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
Kahlenberg, J.M., Lundberg, K.C., Kertesy, S.B., Qu, Y. & Dubyak, G.R. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-κB-driven protein synthesis. J. Immunol. 175, 7611–7622 (2005).
Miao, E.A., Rajan, J.V. & Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 243, 206–214 (2011).
Masters, S.L., Simon, A., Aksentijevich, I. & Kastner, D.L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 27, 621–668 (2009).
Gross, O. et al. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 (2012).
Miao, E.A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Balci-Peynircioglu, B. et al. Expression of ASC in renal tissues of familial mediterranean fever patients with amyloidosis: postulating a role for ASC in AA type amyloid deposition. Exp. Biol. Med. (Maywood) 233, 1324–1333 (2008).
Shi, C.S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255–263 (2012).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011).
Dupont, N. et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 30, 4701–4711 (2011).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Shmagel, K.V. & Chereshnev, V.A. Molecular bases of immune complex pathology. Biochemistry (Mosc.) 74, 469–479 (2009).
Kahlenberg, J.M., Carmona-Rivera, C., Smith, C.K. & Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 190, 1217–1226 (2013).
Garcia-Calvo, M. et al. Purification and catalytic properties of human caspase family members. Cell Death Differ. 6, 362–369 (1999).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Masters, S.L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Tanaka, N. et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 63, 3625–3632 (2011).
Nakagawa, K. et al. Somatic NLRP3 mosaicism in Muckle-Wells syndrome. A genetic mechanism shared by different phenotypes of cryopyrin-associated periodic syndromes. Ann. Rheum. Dis. 10.1136/annrheumdis-2013-204361 (10 December 2013).
Hoffman, H.M. et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 364, 1779–1785 (2004).
Hoffman, H.M. et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 58, 2443–2452 (2008).
Lachmann, H.J. et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360, 2416–2425 (2009).
Adamczak, S. et al. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J. Neurosurg. 117, 1119–1125 (2012).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267, 2000–2003 (1995).
Fernandes-Alnemri, T. & Alnemri, E.S. Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 442, 251–270 (2008).
Acknowledgements
We thank E. Latz (Institute of Innate Immunity) for immortalized ASC-mCherry, ASC-deficient and NLRP3-deficient mouse bone marrow macrophages and for the expression vector encoding red fluorescent protein–tagged ASC; V. Dixit (Genentech) for Pycard−/− mice; M.C. Baños and J.J. Martínez for technical assistance with both molecular and cellular analyses; L. Martinez-Alarcón for extraction of blood from healthy donors; M. Martínez-Villanueva for analysis of CRP; and C. de Torre for support with proteomics. Supported by the Wellcome Trust (V.C.), Instituto Salud Carlos III (CD12/00523 and CD13/00059 to F.M.-S. and J.A.-I.), Instituto Salud Carlos III–Fondo Europeo de Desarrollo Regional (EMER07/049, PS09/00120 and PI13/00174 to P.P. and PS09/01182 to J.Y.), Fundación Séneca (11922/PI/09 to P.P.) and Fundación Mutua Madrileña (ID98FMM013 to A.B.-M.).
Author information
Authors and Affiliations
Contributions
A.B.-M., F.M.-S., A.I.G., C.M.M., J.A.-I., V.C. and M.B.-C., execution of experiments; J.Y., E.R.-O., J.A., S.B., I.C. and J.I.A., provision of human samples and mutant mice; A.B.-M., F.M.-S., A.I.G., V.C., D.B., J.I.A. and P.P., analysis and interpretation of data; A.B.-M., F.M.-S., V.C., D.B., J.I.A. and P.P., manuscript preparation; and P.P., conception, design and supervision of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 597 kb)
Supplementary Table 1
Genotypes and serum inflammatory markers of patients with inherited autoinflammatory syndromes. (PDF 195 kb)
NLRP3 activation induces the release of ASC particles.
Time-lapse fluorescence microscopy of immortalized mouse BMDMs expressing ASC-mCherry primed in coverslips with LPS (1 μg/ml, 2h) and then perfused with nigericin (20 μM) with a flow rate of 1 ml/min. Frames are recorded every 20 sec, the real length of the video corresponds to the 10 min where the ASC speck is formed and released. Nigericin flow was opened after 2 min of basal recording and from that point nigericin concentration gradually increased in the recording chamber at the flow rate used. In these conditions ASC speck formation was observed after 6 min of nigericin flow opening and the ASC speck release occurred 5 min after formation. (AVI 182 kb)
Rights and permissions
About this article
Cite this article
Baroja-Mazo, A., Martín-Sánchez, F., Gomez, A. et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15, 738–748 (2014). https://doi.org/10.1038/ni.2919
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2919
This article is cited by
-
Colon-targeted S100A8/A9-specific peptide systems ameliorate colitis and colitis-associated colorectal cancer in mouse models
Acta Pharmacologica Sinica (2024)
-
Biological and clinical roles of IL-18 in inflammatory diseases
Nature Reviews Rheumatology (2024)
-
Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
Nature Reviews Drug Discovery (2024)
-
Pathogenic NLRP3 mutants form constitutively active inflammasomes resulting in immune-metabolic limitation of IL-1β production
Nature Communications (2024)
-
VCP Inhibition Augments NLRP3 Inflammasome Activation
Inflammation (2024)