Abstract
The skin is a highly complex organ interspersed with a variety of smaller organ-like structures and a plethora of cell types that together perform essential functions such as physical sensing, temperature control, barrier maintenance and immunity. In this Review, we outline many of the innate and adaptive immune cell types associated with the skin, focusing on the steady state in mice and men, and include a broad update of dendritic cell function and T cell surveillance.
Similar content being viewed by others
Main
The skin is composed of the epidermis, attached to a basement membrane, underlain by the dermis and a subcutaneous fatty region. Each layer is highly complex and is peppered with an array of structures, such as hair follicles, sweat glands (in humans but not in mice), sebaceous glands, nerves, blood vessels and lymphatics. The epidermis and dermis are, in turn, populated by a variety of cell types that together form an orchestrated defense against invading pathogens. These first two layers provide most of the protection against infection, though mounting evidence also points to a defensive role for the microbial flora that also populate this tissue1,2,3,4. As an organ, the skin is a formidable barrier to infection. To ensure successful control of pathogens, contributions to surveillance and effector function must be provided by many different cell types, from keratinocytes that form the infrastructure of the skin to the many resident and migratory leukocyte populations that specialize in immunity or are multifaceted in their regulation of skin homeostasis. In this Review, we examine the role of some of the important contributors to skin immunity.
Immunity in the skin
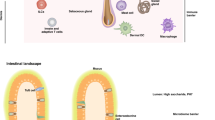
The structure of the epidermis is largely dictated by keratinocytes5, which form an outer enucleated, cornified layer referred to as the stratum corneum (Fig. 1). This is followed by the stratum granulosum containing tight junctions6, then the stratum spinosum and finally the basal layer attached to a complex basement membrane. The stratum corneum and tight junctions within the stratum granulosum ensure an effective physical barrier to the environment and its associated pathogens, whereas the cornified layer also acts as a scaffold for microflora. Recent studies have revealed a vast skin microbiome consisting of bacteria, fungi, viruses and parasites1. This normal flora benefits many aspects of host physiology including wound healing2,3, protection against pathogens3 (Fig. 1) and normal development of the immune system4.
The epidermis comprises several layers of keratinocytes, including two that seal against the outer environment: the stratum corneum composed of corneocytes (which are terminally differentiated from keratinocytes) and the stratum granulosum, a living layer of cells that generate tight junctions between adjacent cells. The skin microflora benefits many aspects of host physiology, including wound healing and protection against pathogens, the latter as a consequence of direct production of antimicrobials, induction of host-cell antimicrobials, conversion of host cell products into antimicrobials or by altering the nature of the adaptive immune response. After skin wounding, for example, keratinocytes can be activated via TLR3 to induce inflammation in response to endogenous RNA released by necrotic cells2,3. Although inflammation is needed for wound healing, overt inflammation can slow the repair process and be harmful. To balance the strong inflammatory response induced via TLR3, lipoteichoic acid produced by Staphylococcus epidermidis, a prominent member of the normal flora, can signal TLR2 to induce TRAF1, which impairs TLR3-dependent production of IL-6 and TNF and limits inflammation and leukocyte recruitment3. Activation of TLR2 by S. epidermidis can also increase production of antimicrobial molecules that defend against pathogens like group A Streptococcus pyogenes115, ensuring both steady-state protection and providing potential defense at wound sites.
Keratinocytes have a key innate role in detection of pathogens and defense7, expressing many pattern recognition receptors, including Toll-like receptors (TLRs) that recognize a wide variety of pathogen components and self components, Nod-like receptors (NLRs) Nod1 and Nod2, which respond to bacterial peptidoglycan and NLR pyrin domain–containing proteins that respond to viral, fungal and self constituents. Expression of RIG-I–like receptors (RLRs) enables response to viral RNA, whereas C-type lectins, such as dectin-1, detect fungal infections. Together, these and other pathogen recognition systems make keratinocytes a formidable barrier for frontline detection of pathogen invasions. In direct response to microbial detection or through indirect activation by cytokines such as interleukin 22 (IL-22), keratinocytes can produce a vast array of microbiocides including antimicrobial peptides such as LL-37 and β-defensins, as well as RNases and S100 family members. The epidermis is also bathed in other proteins that have microbiocidal activity such as dermicidin, produced by eccrine glands (one of the two types of sweat glands present in human but not mouse skin), sebum, a lubricating agent produced by sebaceous glands, and filaggrin, a keratinocyte-derived moisture-retention agent, which can also be cleaved into acids that are capable of slowing pathogen growth1,7,8.
Keratinocytes can also produce chemokines and cytokines in response to pathogenic stimuli, including CXCL9, CXCL10, CXCL11, CCL20, tumor necrosis factor (TNF), IL-1α and IL-1β, IL-6, IL-10, IL-18 and IL-33 (refs. 5,9). Chemokines can be critical in recruiting T cell and innate effectors such as monocytes to the skin10, whereas cytokines arm effectors and direct the immune response to ensure appropriate effector mechanisms11.
In the epidermis there are several specialized cell types that have distinct roles in immunity, including memory αβ T cells12,13,14 and Langerhans cells (epidermal dendritic cells (DCs); Fig. 2). The epidermis also contains γδ T cells, which make up the vast bulk of all epidermal T cells in mice, where they are known as dendritic epidermal T cells (DETCs)15, but only represent a minor subset in the human epidermis16. This region of the skin also contains sparsely dispersed cell types such as Merkel cells, which serve the nervous system17, and melanocytes, which are responsible for protection against ultraviolet light damage.
(a) The mouse epidermis is interspersed with three important immune cell types, Langerhans cells, γδ T cells termed DETCs and CD8+ TRM cells. The dermis contains cells of the immune system including Treg cells, CD4+ TRM cells, CD4+ T effector memory cells (TEM cells), other γδ T cells, ILCs and several populations of DCs. In mice, dermal DCs can be divided into CD11b+ DCs and CD103+ DCs, but data collected in humans suggests that the mouse CD11b+ DCs might contain both a monocyte-derived counterpart (CD14+ DCs in humans) and a pre-DC–derived counterpart (CD1c+ DCs in humans). NK cells are a type of ILC1. (b) Langerhans cells (green) and CD8+ TRM cells (red) in the skin of mice 44 d after infection with HSV-1. (c) DETCs (green) and HSV-1–specific CD8+ TRM cells (red) in the epidermis of mice 30 d after infection with HSV-1. Images in b and c were provided by A. Zaid and S. Mueller.
The dermis is not as densely packed with cells as the epidermis, and is instead extensively composed of elastin fibers, collagen fibers and other extracellular matrix largely produced by fibroblasts. It is interspersed with a number of structures and many different cell types. Blood vessels and capillary beds are spread throughout the dermis, as are nerves that access the dermis and epidermis. Draining lymphatics begin in the dermis and traverse the deeper layers of the skin to eventually access the lymph nodes. Some of the important immunologically relevant cell types in the dermis include mast cells, macrophages, various DC subsets, innate lymphoid cells (ILCs), γδ T cells and αβ T cells (Fig. 2). In the human, the dermis abuts the epidermis in a ridge-like manner, termed glandular ridges, whereas the dermal-epidermal junction in mice is fairly flat except where its many hair follicles form involutions.
Hair follicles consist mainly of keratinocytes and are highly complex structures, continuous with the epidermis, penetrating deep into the dermis18,19. They are formed for life, though some de novo generation has been demonstrated at wound sites20. Hair follicles perform several tasks18,19, the most obvious of which is production of hairs, which can contribute to thermoregulation, sensory activity, camouflage, physical protection and dispersion of sweat, sebum and pheromones. The relative importance of hair follicles in skin immunity is yet to be fully elucidated, but these structures harbor a large proportion of the microbiome, with distinct microbial constituents1, implicating them in the immune system–microbiome cross-talk. Recent evidence shows that different regions of the follicle can express a variety of chemokines in a regulated manner that influences trafficking of immune cells10. Hair follicles are implicated in controlling access of monocytes and Langerhans cells to the epidermis during periods of repopulation, acting as a 'gateway' to the epidermis. Hair follicle–associated expression of chemokines may also control the accumulation of perifollicular cells such as macrophages, mast cells and DCs19.
The skin has a vast range of cell types that contribute to adaptive and innate immune functions. Below we will explore the role of various subsets of lymphocytes and DCs associated with this tissue.
γδ T cells
The mouse DETC γδ T cell population, which express a conserved Vγ5 Vδ1 T cell antigen receptor (TCR), are distributed extensively throughout the epidermis, forming a dense network of dendritic-like cells that are essentially locked in position15 (Fig. 2). Although the ligand of their invariant TCR is unknown, they appear to be signaled by self ligands in the steady state, constitutively clustered on dendrites that form polarized anchors at epithelial tight junctions21. This process is likely important for monitoring epidermal integrity. DETCs are radioresistant22, exibiting slow homeostatic division under steady-state conditions21 and requiring expression of IL-2Rβ (ref. 23) and access to exogenous IL-15 for proliferation and survival24. They use an array of molecules to monitor 'stress' in the epidermis25. These molecules include the receptor NKG2D, which detects ligands such as Rae-1, upregulated during viral infection or tumorogenesis26, TLRs that monitor for foreign ligands as well as self ligands, the junctional adhesion molecule family member JAML, which binds the epithelial coxsackie virus and adenovirus receptor (CAR)27 that is potentially revealed when integrity of the tight junction is compromised, and CD100 (ref. 28), which recognizes plexin β2, upregulated on keratinocytes after wounding. DETCs have primarily been shown to participate in wound healing29 but can contribute to inflammation (also essential for wound healing) and tumor surveillance26, and may participate in contact hypersensitivity and antibacterial responses15. Without DETCs, wounds show impaired keratinocyte proliferation, reduced inflammation and slower closure29. How DETCs exert their repair function is not fully elucidated, but they can induce keratinocyte deposition of hyaluronan, which recruits wound-repair macrophages30, and they produce keratinocyte growth factors such as insulin-like growth factors. Activated DETCs can express a variety of chemokines and cytokines including CCL3, CCL4, CCL5 and XCL1 (ref. 31), IFN-γ and IL-2 (ref. 32) and IL-13 (ref. 33), which potentially contribute to inflammation and recruitment of innate and adaptive immune cell types as well as to eliciting production of IgE33. Like mouse DETCs, γδ T cells present in human epidermis appear to contribute to wound healing16.
In addition to the presence of DETCs in the epidermis, mice have a largely Vγ5−Vγ4+ population of γδ T cells in the dermis22. Expression of eGFP under the Cxcr6 promoter in mice deficient of αβ T cells allowed the visualization of DETCs in the epidermis but also revealed a separate population of γδ T cells in the dermis, often associated with major histocompatibility complex (MHC) class II+ cells. Unlike DETCs, the dermal γδ T cell population was not dependent on IL-15 for survival but required IL-7. γδ T cells had been reported to enhance CD4+ T cell immunity to some infections, such as with Bacillius Calmette-Guerin (BCG)25, and this appears to be facilitated by the production of IL-17 by dermal γδ T cells, leading to neutrophil recruitment, which may contribute by delivering antigen to the draining lymph node22. Notably, mice lacking this population of γδ T cells showed reduced dermatitis associated with application of imiquimod34,35, whereas psoriasis patients showed increased numbers of IL-17–producing γδ T cells in their skin36.
Innate lymphoid cells
Despite extensive characterization of ILCs in tissues such as the spleen, mesenteric lymph nodes and lungs37, ILCs other than NK cells have only recently been identified in the skin35,38,39. In other organs, three major classes of ILCs have been distinguished, referred to as ILC1, ILC2 and ILC3 (ref. 37). ILC1 populations express the transcription factor T-bet, make IFN-γ and consist of conventional NK cells as well as nonlytic populations. This group of ILCs participate in viral immunity and are triggered by the cytokines IL-12 and IL-18. ILC2 populations, which include members referred to as nuocytes and natural helper cells, express the transcription factor GATA-3, respond to the cytokines IL-25 and IL-33 and produce the TH2 cell–type cytokines IL-5, IL-9 and IL-13 and amphiregulin. They have roles in tissue repair, asthma and responses to helminths. ILC3 populations include lymphoid-tissue-inducer lymphocytes, express the transcription factor RORγt and produce cytokines such as IL-22, IL-17A and some IFN-γ, responding to IL-1β and IL-23 and participating in responses to bacteria, tissue repair and development of lymphoid tissue37.
Of the ILC1 subset, only NK cells have so far been detected in healthy skin, usually in small numbers that are increased in conditions such as psoriasis40,41,42. ILC2 populations in other (nonskin) tissues are defined as lineage-negative cells that express CD45, CD90, CD127 and ICOS, with variable expression of c-Kit, Sca-1 and the IL-33 receptor ST2. IL-13, produced by the ILC2 population, can function by suppressing monocyte synthesis of inflammatory cytokines (for example, IL-6, IL-1β and TNF)43, by inducing mucous production by globlet cells (not relevant to the skin)44 and by recruiting eosinophils indirectly via eliciting eotaxin and IL-5 from other cell types such as epithelial cells45. ILC2-like cells, recently recognized in mouse and human skin38,39, express CD25 and ST2, constitutively produce IL-13, require IL-7 for survival and are activated by thymic stromal lymphopoietin (TSLP), which increases their production of IL-13 and induces IL-4. ILC2 cells were found to accumulate in patients with atopic dermatitis, a TH2 cell–like allergic disease, whereas in T cell–deficient mice, depletion of the ILC2 population with anti-CD25 monoclonal antibody reduced inflammation associated with TH2 cell–type cytokines in a dermatitis model38. In the steady state, the skin ILC2 population was often found to associate with mast cells as they trafficked through the dermis39. Constitutive production of IL-13 by this ILC2 population suppressed the function of mast cells. However, in inflammatory conditions in which mice were injected with an IL-2 complex that stimulated CD25 receptors, these innate lymphocytes were more frequent and increased their production of IL-5 and IL-13 and the recruitment of eosinophils. These findings suggest that ILC2 can have both suppressive or inflammatory effects depending on the conditions and cellular targets39. Finally, ILC3-like cells have been identified in mouse skin under inflammatory conditions and might contribute to an experimental psoriatic skin condition induced by administration of imiquimod35.
Dendritic cells
DCs are present throughout the body and can be divided into at least five broad groups based on phenotypic, functional and developmental analysis (Table 1). Such diversity is probably needed to cover the vast array of pathogens encountered by the immune system, a conclusion supported by the differential expression of pathogen recognition receptors on DC subgroups46,47,48. These groups include plasmacytoid DCs, migratory and lymphoid tissue-resident CD8+ DC–like DCs, migratory and lymphoid tissue–resident CD11b+ DCs, Langerhans cells and monocyte-derived DCs. The different groups of DCs are derived from a range of progenitors49. Fetal liver–derived and yolk sac–derived cells generate Langerhans cells during embryonic development50,51, whereas common DC progenitors (CDPs) seed plasmacytoid DCs and other conventional DC subsets. Pre-DCs, which are downstream of CDPs, have lost plasmacytoid DC generation capacity but generate lymphoid tissue–resident CD8+ DCs and CD11b+ DCs52 and probably their migratory counterparts, referred to as respective CD103+ and CD11b+ DCs, found in many tissues53,54. Plasmacytoid DCs can also be derived from lymphoid-primed multipotent progenitors (LMPPs), which are of bone marrow origin55. Though their exact contribution to the steady-state pool of DCs is unclear, blood monocytes in adult mice can also generate DCs in various tissues, including the lungs56, skin57, gut58 and spleen59, usually but not strictly under inflammatory conditions. Such monocyte-derived DCs can contribute to both innate59 and adaptive immune responses60.
DC subsets in the skin have been extensively studied in mice and humans53,54,61,62, and a picture is beginning to form that is relatively similar between species. A very simple view of skin DCs in mice is that there are three distinct subsets54: Langerhans cells, found in the epidermis, and two dermal populations corresponding to the CD11b+ DCs and CD103+ DCs found in other organs53. Based on several findings that suggest CD11b+ DCs in various tissues, including the skin, are heterogeneous48,53,58, it is likely that dermal CD11b+ DCs consist of two different populations, which are phenotypically similar for most surface markers; one group deriving from pre-DCs and similar to splenic CD8− DCs and the other derived from monocytes. This would match nicely with DCs in human skin, which comprise up to four different subsets: Langerhans cells, CD1c+ DCs, CD14+ DCs and a recently identified CD141+ DC subset63. In this scheme, Langerhans cells are found in both species, whereas human CD141+ DCs match mouse CD103+ dermal DCs and human CD1c+ DCs of pre-DC origin and CD14+ DCs of monocyte origin would together make up the mouse CD11b+ dermal subset (Fig. 2). Our rapidly advancing capacity to define common relationships between mouse and human DC subsets will aid clinical application.
CD103+ dermal DCs64,65,66 and their human equivalents, the CD141+ population63 are members of the broadly represented CD8+ DC–like DC group, with properties aligned to mouse splenic CD8+ DCs. This group of DCs has a relatively well-defined role in immunity, particularly important for CD8+ T cell responses and viral immunity54. These DCs are highly adept at capturing dead cells, express molecules directed to recognition of viral or other intracellular pathogens, express IL-12 (at least in mice) to drive TH1 cell–type immunity and are very efficient at cross-presenting antigens for induction of CD8+ T cell responses67. CD8-like DCs express molecules such as Clec9A, which recognizes F-actin released from necrotic cells68,69, and TLR3 (ref. 47), which binds double-stranded RNA, an intermediate associated with most viral infections, indicating a major focus on intracellular, primarily viral, pathogens. For herpes simplex virus-1 (HSV) infection of the skin, CD103+ DCs were found to efficiently present viral antigens to both CD4+ T cells and CD8+ T cells, supporting their role in viral immunity70. They were also the dominant subset capable of cross-presenting skin-associated self antigens to CD8+ T cells62,70, indicating a potential role in self tolerance in the steady state. Human dermal CD141+ DCs also efficiently cross-present antigens63, aligning their function with the mouse CD103+ DC subset and supporting a role for these DCs in viral immunity.
The role of migratory CD11b+ DCs in mouse skin immunity is not as clear, though in other tissues they have been linked to TH17 cell–mediated immunity71,72. After skin infection with HSV, CD11b+ DCs were shown to present antigens preferentially to CD4+ T cells70, whereas after intradermal injection of protein antigen in adjuvant these DCs stimulated CD4+ T cells in the lymph node (LN)73 and skin74, in the latter case also providing antigen for regulatory T cells (Treg cells), which dampened responses by IFN-γ-producing CD4+ T cells74. Intradermal infection with Leishmania major has also revealed an important role for CD11b+ DCs in antigen presentation75. As indicated earlier, the CD11b+ dermal DC group may contain two different subsets. One study divided the CD11b+ dermal DCs based on expression of aldehyde dehydrogenase activity (ALDH), showing that ALDH+ DCs could convert retinol to retinoic acid that could drive Treg cell development76, mirroring what had already been shown for ALDH+ DCs in the gut77.
Langerhans cells are positioned in the epidermis above the basal keratinocytes and are able to project their dendrites upward toward the cornified epithelial layer6. These dendrites can pass through tight junctions in a regulated manner, enabling sampling of material within the cornified layer without breaching epidermal integrity6,78. The function of Langerhans cells has been intensively studied in the last decade, with several reviews comprehensively covering this work79,80,81. These DCs were originally considered the archetypal DCs responsible for priming skin immunity but a few years ago were shown to be of questionable importance for responses to contact allergens or herpes simplex virus82,83. More recently it has been shown that Langerhans cells may have a role in contact hypersensitivity when the sensitizing agent is delivered at a very shallow depth, excluding access by deeper dermal DCs80. More recently, strong evidence was provided that Langerhans cells contribute to priming immunity to skin pathogens such as yeast (Candida albicans) and bacteria (Staphylococcus aureaus), favoring induction of TH17 cell responses important for these pathogens84,85. In contrast, Langerhans cells had a poor capacity to induce CD8+ T cell immunity, which was attributed to CD103+ DCs. Langerhans cells can sample bacterial toxins on the apical side of tight junctions, enabling the generation of humoral immunity critical for protection from the damaging effects of this toxin, without requiring free toxin to breach the epithelial barrier78. Langerhans cells were also reported to be immunosuppressive, either inducing T cell deletion86, or activating Treg cells that dampened skin responses87. In the presence of the pathogen C. albicans, human Langerhans cells stimulated both skin-associated Treg cells and effector T cells, the latter producing IL-17 and IFN-γ (ref. 88). Together, the above findings support a model where Langerhans cells provide important regulatory feedback but can also selectively contribute to effector T cell responses.
Functionally, plasmacytoid DCs are only moderately efficient antigen presenting cells compared to conventional DCs but can be a major source of type I interferons, especially during viral infection89. These atypical DCs circulate in the blood and are essentially absent from normal human skin90. However, they may be recruited during inflammatory conditions such as viral infection, allergy or autoimmunity. In the autoimmune disease systemic lupus erythematosis (SLE), they have an important role, producing IL-6 and type I interferons in response to autoantibody–nucleic acid complexes, driving autoreactive B cell responses91. In psoriasis, they drive disease initiation by producing type I interferon in response to complexes of the antimicrobial peptide LL37 bound to self nucleic acids92.
Before leaving the discussion of DCs, it is important to emphasize that migratory DCs present in the skin are not the only DCs involved in skin immunity. Resident DC populations in the cutaneous draining LN include a CD11b+ DC population and the CD8α+ DCs, both of which also exist in the spleen. There is limited evidence that LN-resident CD11b+ DCs participate in immunity93,94,95, whereas LN-resident CD8α+ DCs have been implicated in responses to various organisms, particularly viruses67. In studies examining access of CD8α+ DCs to HSV-1 antigen, migratory dermal DCs were implicated in the transport of antigen eventually passed on to CD8α+ DCs for cross-presentation96. Just how extensively LN-resident populations contribute to skin immunity probably depends on the pathogen and may be especially important for those infectious agents that kill migratory DCs.
αβ T cells
Human skin contains a large number of αβ T cells, essentially all of memory phenotype, and in number nearly twice that found in the blood13. These memory T cells consist of both CD4+ and CD8+ subsets, with about 10% of the CD4+ T cells expressing Foxp3, indicative of a Treg cell phenotype88. Some researchers have referred to all the T cells in human skin as tissue-resident memory cells, but we prefer to distinguish between those cells simply passing through the tissue (either recirculating memory cells or effector cells) and those that are disconnected from the greater blood circulation, remaining permanently in the tissue as a memory population; these latter cells we call tissue-resident memory cells (TRM cells)12. The idea of TRM cells in human skin was suggested by studies of psoriasis, where normal healthy looking 'uninvolved' skin from patients was transplanted onto immunocompromised mice that later developed graft psoriasis because of the presence of passenger T cells97. Failure to detect human T cells in the circulation of grafted mice suggested T cells resided permanently in the grafts. Detection of large numbers of T cells in human skin, expressing homing receptors for this tissue, was used as further support for the existence of TRM cells13. In the mouse, studies of HSV-1 infection12,98 demonstrated that skin-associated T cells could exist as a population disconnected from the wider circulation. First, greater numbers of memory CD8+ T cells were detected in areas of previous infections. Second, these memory T cells were maintained in skin grafts. Finally, when virus-specific T cells from male mice were transferred into female mice that were then infected with HSV-1, responding cells from the male mouse were rejected from the circulation by their female hosts but survived in the epidermis. These observations showed that CD8+ T cells could accumulate in the epidermis as a long-term tissue-resident memory cell population and, as an aside, implied that the epidermis was somehow disconnected from the greater circulatory pool; otherwise host T cells would have rejected the transferred virus-specific male CD8+ T cells located there. Following demonstration of TRM cells in skin and neuronal ganglia12, T cells of a similar phenotype were reported to reside permanently in a wealth of tissues, including the gut, brain, salivary glands, the female reproductive tract, lungs and elsewhere99,100,101,102,103,104, supporting earlier studies that described T cells with limited recirculation potential in the brain and gut105. In most studies, TRM cells were of the CD8+ T cell lineage and expressed a unique set of markers, including CD69, VLA-1 and CD103 (ref. 12). For skin TRM cells, CD69 was expressed independent of antigen recognition, as was CD103 (ref. 106), though antigen recognition was required for CD103 expression in tissues such as neuronal ganglia106 and the brain107. TRM cells were also reported to develop from CD4+ T cells, in this case associated with lung infection99.
In a study of HSV-1 infection of the skin98, memory CD4+ T cells and CD8+ T cells showed different tissue locations and recirculation patterns, with CD8+ TRM cells found resident in the epidermis and memory CD4+ T cells preferentially found in the dermis but capable of recirculating through the blood (Fig. 3). Evidence for both CD4+ skin TRM cells and CD8+ skin TRM cells came from examination of humans treated with the T cell–depleting monoclonal antibody alemtuzumab (anti-CD52), which revealed a major cohort of T cells in patients' skin, despite depletion of essentially all T cells from the peripheral blood circulation108. This not only demonstrated the existence of human CD4+ TRM cells and CD8+ TRM cells as well as tissue-resident Foxp3+ Treg cells, but hinted that the surviving TRM cells provided functional immunity, as patients showed no increase in susceptibility to infections. Such capacity of TRM cells to control infections is consistent with other studies showing that CD8+ TRM cells protect mice against skin infection with viruses12,109. Further support for skin CD4+ TRM cells was provided by a study that used T cells expressing the photoconvertible fluorescent protein Kaede to track migration110. By using ultraviolet light to convert skin-associated T cells expressing Kaede from green to red, a proportion of CD4+ T cells were shown to migrate into the circulation, while many remained in the skin and expressed markers of TRM cells, i.e., CD103 and CD69. Consistent with this report, about half the skin-associated CD4+ T cells in mice110 and humans13 lack expression of CCR7, a chemokine receptor required for migration to the draining LN111,112.
After resolution of skin infection, various populations of memory T cells can be recognized in the skin and wider circulation. These include CD8+ TRM cells that are localized to the epidermis at the original site of infection, are rare in distant skin sites and are absent from the circulation. For CD4+ T cells there appear to be skin-resident CD4+ TRM cells in the dermis as well as a circulating memory cell population (traditionally termed T effector memory cells (TEM cells), although they could be a distinct, recirculating memory T cell subset110) found in both the dermis and wider circulation. It is yet to be determined whether CD4+ TRM cells are more highly represented at sites of previous infection as speculated in this diagram. Within the lymphoid tissues and blood, there are central memory CD4+ T cells (CD4+ TCM cells), CD8+ TCM cells and CD8+ TEM cells, none of which appear to enter the dermis during memory, and CD4+ TEM cells, which can recirculate to the dermis. Although CD8+ TRM cells can enhance protection against local infection12, they may recruit circulating memory cells CD8+ T cells (TCM cells and/or TEM cells) to achieve this function104.
Although within mouse skin CD4+ T cells appear to exist as both recirculating and resident populations located primarily in the dermis, memory CD8+ T cells are predominantly resident and restricted to the epidermis14,97,98,110 (Fig. 3). A few CD8+ T cells can be found in the dermis in both humans and mice, but many of these are likely to be effector-type populations. The latter persist for about a month after the resolution of local infection in mice, after which they are confined to the epidermis around the site of their original recruitment12,98. Similarly, CD8+ T cells were recently shown to vanish from the dermis 2 weeks after resolution of HSV-2 infection in humans, retreating to the dermal-epidermal junction14. The surviving cells had TRM cell hallmarks: persisting for long periods in a highly localized pattern, expressing effector molecules associated with cytotoxicity and downregulating CCR7 and a G protein–coupled receptor S1P1 required for tissue egress. Notably, they also carried the αα form of the CD8 molecule14. Given that HSV-2 infections in humans are often associated with continuous virus reactivation113, the possibility that ongoing antigen stimulation drives this form of CD8 expression requires further investigation.
Infection with vaccinia virus showed that protective TRM cells were also focused around the original site of infection as well as revealing a level of seeding throughout the broader noninfected areas of the skin109. This is similar to TRM cell lodgment in the gut after systemic virus infection101. The detectable but relatively poor seeding away from sites of active infection probably reflects low inflammatory chemokine expression in the steady state because localized inflammation or chemokine administration efficiently seeds TRM cells into the skin and mucosal tissues in the absence of antigen106,114. Although the frequency of TRM cells at sites distant from the initial infection can be relatively low12,109, their numbers in the skin increase dramatically after repeated immunization109. Use of parabiotic mice demonstrated efficient protection by TRM cells compared to circulating memory CD8+ T cells109. Finally, TRM cells may protect in part by recruiting circulating memory cells to sites of virus reactivation or reinfection104, though they also have direct effector capabilities14,107.
Concluding remarks
The skin is rich in immune cells and infrastructure to ensure our fragile underbodies are well protected from an otherwise hostile environment. The last few years have seen a rapid increase in our knowledge of the cell types associated with the skin and have led to the identification of an ever-expanding array of cytokines, chemokines, antimicrobial peptides and other players that can be added to the immune arsenal. The exciting interplay between the host's immune system and normal flora and the influence this has on pathogen control is just beginning to be elucidated. It is evident that DCs are much more diverse than was first anticipated and that environmental cues as well as subset-specific genetic programs ensure the right DC is available to tailor immunity to each invading pathogen and to ensure rigorous control of self tolerance. Keratinocytes have a major role in pathogen detection, as do ILCs, γδ T cells, DCs and mast cells. Together, these cells direct and drive the appropriate T helper cell responses to mediate pathogen control and the development of memory T cells that control recurrent infections. Although circulating memory T cells are important immune mediators, it is the newly recognized tissue-resident memory T cells that provide premium protection and offer new strategies for vaccination. Knowing how these cells develop and expand within the skin and how we can preferentially draft them via vaccination will be important for controlling skin-associated pathogens.
References
Grice, E.A. & Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 9, 244–253 (2011).
Cavassani, K.A. et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 205, 2609–2621 (2008).
Lai, Y. et al. Commensal bacteria regulate Toll-like receptor 3–dependent inflammation after skin injury. Nat. Med. 15, 1377–1382 (2009).
Naik, S. et al. Compartmentalized control of skin Immunity by resident commensals. Science 337, 1115–1119 (2012).
Nestle, F.O., Di Meglio, P., Qin, J.-Z. & Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Kubo, A., Nagao, K., Yokouchi, M., Sasaki, H. & Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206, 2937–2946 (2009).
Kuo, I.-H., Yoshida, T., De Benedetto, A. & Beck, L.A. The cutaneous innate immune response in patients with atopic dermatitis. J. Allergy Clin. Immunol. 131, 266–278 (2013).
Brown, S.J. & McLean, W.H.I. One remarkable molecule: filaggrin. J. Invest. Dermatol. 132, 751–762 (2012).
Meephansan, J., Tsuda, H., Komine, M., Tominaga, S.-i. & Ohtsuki, M. Regulation of IL-33 expression by IFN-γ and tumor necrosis factor-α in normal human epidermal keratinocytes. J. Invest. Dermatol. 132, 2593–2600 (2012).
Nagao, K. et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 13, 744–752 (2012).This study provided important insight into production of chemokines by different regions of hair follicles and implicated some of these in the recruitment of monocytes and Langerhans cells into the epidermis. This work raised the idea that hair follicles may act as a gateway to the epidermis.
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).Described is a non-recirculating form of tissue-resident memory CD8+ T cells, which was the first evidence that these cells could provide local protection against infection.
Clark, R.A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Zhu, J. et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497, 494–497 (2013).
MacLeod, A.S. & Havran, W.L. Functions of skin-resident γδ T cells. Cell Mol. Life Sci. 68, 2399–2408 (2011).
Toulon, A. et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206, 743–750 (2009).
Maricich, S.M. et al. Merkel cells are essential for light-touch responses. Science 324, 1580–1582 (2009).
Schneider, M.R., Schmidt-Ullrich, R. & Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 19, R132–R142 (2009).
Paus, R. & Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 341, 491–497 (1999).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Chodaczek, G., Papanna, V., Zal, M.A. & Zal, T. Body-barrier surveillance by epidermal γδ TCRs. Nat. Immunol. 13, 272–282 (2012).This work provided evidence that γδ T cells of the epidermis (referred to as dendritic epidermal T cells or DETCs) are constitutively signaled through their TCR via polarized interactions anchored in epidermal regions associated with tight junctions. These interactions are thought to monitor steady-state integrity of the epidermis. This report also provides some outstanding long-term live imaging of DETCs, showing their dendrite orientation and cellular replication.
Sumaria, N. et al. Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J. Exp. Med. 208, 505–518 (2011).
Ye, S.K. et al. Differential roles of cytokine receptors in the development of epidermal gamma delta T cells. J. Immunol. 167, 1929–1934 (2001).
De Creus, A. et al. Developmental and functional defects of thymic and epidermal V gamma 3 cells in IL-15–deficient and IFN regulatory factor-1–deficient mice. J. Immunol. 168, 6486–6493 (2002).
Hayday, A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Girardi, M. et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 294, 605–609 (2001).
Witherden, D.A. et al. The Junctional Adhesion Molecule JAML Is a Costimulatory Receptor for Epithelial T Cell Activation. Science 329, 1205–1210 (2010).
Witherden, D.A. et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity 37, 314–325 (2012).
Havran, W.L., Chien, Y.H. & Allison, J.P. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science 252, 1430–1432 (1991).
Jameson, J.M., Cauvi, G., Sharp, L.L., Witherden, D.A. & Havran, W.L. γδ T cell-induced hyaluronan production by epithelial cells regulates inflammation. J. Exp. Med. 201, 1269–1279 (2005).
Boismenu, R., Feng, L., Xia, Y.Y., Chang, J.C. & Havran, W.L. Chemokine expression by intraepithelial γδ T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J. Immunol. 157, 985–992 (1996).
Matsue, H., Cruz, P.D., Bergstresser, P.R. & Takashima, A. Profiles of cytokine mRNA expressed by dendritic epidermal T cells in mice. J. Invest. Dermatol. 101, 537–542 (1993).
Strid, J., Sobolev, O., Zafirova, B., Polic, B. & Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 334, 1293–1297 (2011).
Gray, E.E. et al. Deficiency in IL-17–committed Vγ4+ γδ T cells in a spontaneous Sox13-mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat. Immunol. 14, 584–592 (2013).
Pantelyushin, S. et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122, 2252–2256 (2012).
Cai, Y. et al. Pivotal role of dermal IL-17–producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011).
Philip, N.H. & Artis, D. New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol. Cell Biol. 91, 225–231 (2013).
Kim, B.S. et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170ra116 (2013).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573 (2013).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Batista, M.D. et al. Skewed distribution of natural killer cells in psoriasis skin lesions. Exp. Dermatol. 22, 64–66 (2013).
Ebert, L.M., Meuter, S. & Moser, B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J. Immunol. 176, 4331–4336 (2006).
Minty, A. et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature 362, 248–250 (1993).
Cohn, L. et al. Th2-induced airway mucus production is dependent on IL-4Rα, but not on eosinophils. J. Immunol. 162, 6178–6183 (1999).
Pope, S.M. et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J. Allergy Clin. Immunol. 108, 594–601 (2001).
Segura, E. et al. Differential expression of pathogen-recognition molecules between dendritic cell subsets revealed by plasma membrane proteomic analysis. Mol. Immunol. 47, 1765–1773 (2010).
Edwards, A.D. et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33, 827–833 (2003).
Miller, J.C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Belz, G.T. & Nutt, S.L. Transcriptional programming of the dendritic cell network. Nat. Rev. Immunol. 12, 101–113 (2012).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac–derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Naik, S.H. et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7, 663–671 (2006).
Ginhoux, F. et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 (2009).This report shows that CD103+ DCs exist in many tissues, and provides important information about the origin of these DCs and their CD11b+ counterparts in the same tissues, showing the latter are heterogeneous in origin.
Heath, W.R. & Carbone, F.R. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 10, 1237–1244 (2009).
Onai, N. et al. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity 38, 943–957 (2013).
Jakubzick, C. et al. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J. Immunol. 180, 3019–3027 (2008).
Ginhoux, F. et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7, 265–273 (2006).
Farache, J., Zigmond, E., Shakhar, G. & Jung, S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol. Cell Biol. 91, 232–239 (2013).
Serbina, N.V., Salazar-Mather, T.P., Biron, C.A., Kuziel, W.A. & Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Wakim, L.M., Waithman, J., van Rooijen, N., Heath, W.R. & Carbone, F.R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Henri, S. et al. Disentangling the complexity of the skin dendritic cell network. Immunol. Cell Biol. 88, 366–375 (2010).
Henri, S. et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 207, 189–206 (2010).
Haniffa, M. et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37, 60–73 (2012).This report identifies the human skin counterpart of the mouse CD103+ dermal DCs.
Poulin, L.F. et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 204, 3119–3131 (2007).
Bursch, L.S. et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204, 3147–3156 (2007).
Ginhoux, F. et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204, 3133–3146 (2007).
Shortman, K. & Heath, W.R. The CD8+ dendritic cell subset. Immunol. Rev. 234, 18–31 (2010).
Zhang, J.-G. et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 36, 646–657 (2012).
Ahrens, S. et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 36, 635–645 (2012).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 (2009).
Schlitzer, A. et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 (2013).
Persson, E.K. et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969 (2013).
Itano, A.A. & Jenkins, M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4, 733–739 (2003).
McLachlan, J.B., Catron, D.M., Moon, J.J. & Jenkins, M.K. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 30, 277–288 (2009).
Ritter, U., Meissner, A., Scheidig, C. & Korner, H. CD8α- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 34, 1542–1550 (2004).
Guilliams, M. et al. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood 115, 1958–1968 (2010).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF- and retinoic acid dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Ouchi, T. et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 208, 2607–2613 (2011).
Kaplan, D.H. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 31, 446–451 (2010).
Romani, N., Brunner, P.M. & Stingl, G. Changing views of the role of Langerhans cells. J. Invest. Dermatol. 132, 872–881 (2012).
Igyarto, B.Z. & Kaplan, D.H. Antigen presentation by Langerhans cells. Curr. Opin. Immunol. 25, 115–119 (2013).
Allan, R.S. et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 (2003).
Kaplan, D.H., Jenison, M.C., Saeland, S., Shlomchik, W.D. & Shlomchik, M.J. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620 (2005).
Igyarto, B.Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).This report is one of the first to provide convincing evidence that Langerhans cells have a function in antigen presentation and priming of T cells to infectious agents. It provides evidence that Langerhans cells induce T H 17 cell–mediated immunity to C. albicans . It also shows that Langerin+ (CD103+) DCs are important for T H 1 cell and cytotoxic T cell responses.
Haley, K. et al. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J. Immunol. 188, 4334–4339 (2012).
Shklovskaya, E. et al. Langerhans cells are precommitted to immune tolerance induction. Proc. Natl. Acad. Sci. USA 108, 18049–18054 (2011).
Kautz-Neu, K. et al. Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 208, 885–891 (2011).
Seneschal, J., Clark, R.A., Gehad, A., Baecher-Allan, C.M. & Kupper, T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 36, 873–884 (2012).
Villadangos, J.A. & Young, L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 (2008).
Nestle, F.O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 (2005).
Banchereau, J., Pascual, V. & Type, I. Interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Di Meglio, P., Perera, G.K. & Nestle, F.O. The multitasking organ: recent insights into skin immune function. Immunity 35, 857–869 (2011).
Iezzi, G. et al. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J. Immunol. 177, 1250–1256 (2006).
Itano, A.A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Allan, R.S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004).
Gebhardt, T. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011).Provides clear evidence that CD8+ T cells do not form a recirulating memory cell population that traffics through the skin and instead form resident memory cells in the epidermis. This contrasts CD4+ memory T cells, which do form a recirculating population that traffics through the dermis, lymph nodes, spleen and blood. These recirculating CD4+ memory T cells are shown to have skin tropism.
Teijaro, J.R. et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Anderson, K.G. et al. Cutting Edge: Intravascular Staining Redefines Lung CD8 T Cell Responses. J. Immunol. 189, 2702–2706 (2012).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Casey, K.A. et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875 (2012).
Hofmann, M. & Pircher, H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA 108, 16741–16746 (2011).
Schenkel, J.M., Fraser, K.A., Vezys, V. & Masopust, D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 14, 509–513 (2013).This work shows that tissue-resident memory T cells can recruit circulating memory T cells in response to antigen detection. Thus, the ability of T RM cells to control infection is likely mediated both by their own effector function and their ability to recruit additional circulating effectors.
Klonowski, K.D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004).
Mackay, L.K. et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA 109, 7037–7042 (2012).This study was the first to show that T RM cells can be seeded into tissues (skin and vagina) by inflammation and that this can lead to protection against infection.
Wakim, L.M., Woodward-Davis, A. & Bevan, M.J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA 107, 17872–17879 (2010).
Clark, R.A. et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Science Translational Medicine 4, 117ra117 (2012).This work provides important evidence that human skin may contain non-recirculating T cells, that is, tissue-resident memory T cells, by demonstrating the presence of T cells in skin of patients that have had their circulating T cells depleted by treatment with monoclonal antibody.
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Bromley, S.K., Yan, S., Tomura, M., Kanagawa, O. & Luster, A.D. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J. Immunol. 190, 970–976 (2013).
Bromley, S.K., Thomas, S.Y. & Luster, A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6, 895–901 (2005).
Debes, G.F. et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6, 889–894 (2005).
Wald, A. et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Invest. 99, 1092–1097 (1997).
Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012).
Lai, Y. et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Invest. Dermatol. 130, 2211–2221 (2010).
Acknowledgements
We thank S. Mueller and A. Zaid for the immunohistology in Figure 2b,c. Supported by the Australian Research Council (W.R.H.) and the National Health and Medical Research Council of Australia (W.R.H. and F.R.C.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Heath, W., Carbone, F. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol 14, 978–985 (2013). https://doi.org/10.1038/ni.2680
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2680
This article is cited by
-
Key factors to establish the ovalbumin-induced atopic dermatitis minipig model: age and body weight
Laboratory Animal Research (2022)
-
Tissue-specific immunity in helminth infections
Mucosal Immunology (2022)
-
Nociceptive sensory neurons promote CD8 T cell responses to HSV-1 infection
Nature Communications (2021)
-
Mechanisms of microbe-immune system dialogue within the skin
Genes & Immunity (2021)
-
Dendritic cell migration in inflammation and immunity
Cellular & Molecular Immunology (2021)